NMR HW Key For ibuprofen, below, indicate: a. How many peaks
advertisement

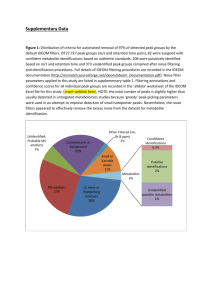
NMR HW Key 1. For ibuprofen, below, indicate: a. How many peaks would appear in the carbon-13 NMR spectrum? b. Show each of the different types of H’s in the structure. For each type of hydrogen in the proton spectrum, indicate how many peaks it would show up as, assuming the N+1 rule works. Ibuprofen 2 peaks 2 peaks 2 peaks Hb 2 peaks Ha CH3 CH3 4 peaks CH CH H3C OH C O CH2 1 peak Hb Ha 2 peaks a. There is symmetry through the 1 and 4 carbons of the benzene ring, so there are only 10 kinds of carbons (4 types of benzene carbons and 6 other carbon types: the 2 isobutyl methyls are also equivalent. b. Starting from the left, the CH3 is two peaks, the CH is 9 peaks, the CH2 is two peaks, Hb is two peaks, Ha is two peaks, the CH is four peaks, the CH3 is two peaks, and the OH is a single peak. 9 peaks 2. Tell precisely how you would use proton NMR spectroscopy to distinguish between the following pairs of compounds. Clearly indicate the number of signals, the number of peaks each signal would have, and/or the locations of signals. Both have three sets of peaks, but the ratio of H’s in cyclohexene is 2:4:4, while in cyclopentene it is 2:4:2. and A O O H3C B CH3 and H3C CH3 3-pentanone has two sets of peaks, due to symmetry, while 2-pentanone has 4 sets of peaks. 1 3. Could you use 13C-NMR spectroscopy to distinguish between the two pairs of strucutres in problem 2? If so, how? If not, why not? Both have three peaks in 13C-NMR. Peak positions should be similar, so it would be difficult to distinguish them. and A O O H3C CH3 3-pentanone has three peaks, due to symmetry, while 2-pentanone has 5 peaks. and B H3C CH3 4. The Proton NMR spectrum of 3-ethoxy-4-hydroxybenzaldehyde is shown below. Answer the questions below on a separate piece of paper. O Ha C O H Hb CH2 CH3 OH Hc a. Which hydrogen(s) produced the peak at 10 ppm? Explain your reasoning. This is the aldehyde H, which normally shows up between 9-10.5 ppm. b. Which hydrogen(s) produced the peaks at 7.5 ppm? Explain your reasoning. These are the peaks for Ha and Hb. Ha should be a singlet and Hb should be a doublet (split by Hc). The sets of peaks overlap. They are close to the aldehyde, so they are further to the left in the aromatic region. c. Which hydrogen(s) produced the peaks at 7.0 ppm? Explain your reasoning. This is Hc. It is a doublet, since it is next to Hb. It is further to the right, since it is adjacent to the OH group. d. Which hydrogen(s) produced the peak at 6.5 ppm? Explain your reasoning. This is the OH hydrogen. It can appear anywhere, and is the only other hydrogen for which there is only one of. e. Which hydrogen(s) produced the peaks at 4.2 ppm? Explain your reasoning. This is the CH2. It is a quartet, since it is adjacent to the methyl. It is at 4.2 ppm since it is attached to an oxygen. f. Which hydrogen(s) produced the peaks at 1.4 ppm? Explain your reasoning. This is the methyl. It is a triplet since it is adjacent to the CH2. It is at 1.4 ppm, since it is an alkane type hydrogen. 2 5. The 13C-NMR spectrum of 3-ethoxy-4-hydroxybenzaldehyde is shown below. O H C O H OH H CH2 CH3 H Answer the questions below on a separate piece of paper. a. Where is the peak for the aldehyde carbon? Explain your reasoning. This is the peak at about 190 ppm. Carbonyls occur at > 160 ppm usually. b. Where are the peaks for the benzene carbons? Explain your reasoning. These are the peaks between 155105 ppm, which is in the typical region for aromatic carbons. c. Where is the peak for the CH2? Explain your reasoning. This is the peak at 65 ppm, which is in the region for a carbon bonded to an electronegative atom. d. Where is the peak for the CH3? Explain your reasoning. This is the peak at 15 ppm, which is in the typical region for an alkane carbon (0-50ppm). 3 6. Propose a reasonable structure for the following set of spectra. Explain what information you got from each spectrum, and how you put it all together to come up with the final structure. Proton NMR Spectrum for question 6 A quartet at 4.4 ppm integrates for 1H. The doublet at 1.6 ppm integrates for 3H. Since these are coupled, we probably have a CH-CH3 unit. The CH is probably bonded to an electronegative atom. The 3H peak at 2.4 ppm is a singlet, so it an isolated CH3 group. Mass Spectrum for question 6 There are two peaks at m/z = 96 and 98, in a 3:1 ratio, so there is probably a Cl. The base peak is at 43, which could be CH3CO+. 4 13 C-NMR spectrum for question 6 (there are no solvent peaks) There are four peaks, for four types of C. The peak near 200 ppm is a C=O. The peak at 60 could be a C-Cl carbon. The other two peaks are alkane type carbons. From proton NMR, we have a CH-CH3 and an isolated CH3. From MS, we have a Cl. From Carbon NMR, we have 4 types of C, one of which is a C=O. Adding up masses: CH-CH3 = CH3 = Cl = C=O = Total = 28 15 35 28 96 There is only one way to put the pieces together that works: H3C O Cl C CH CH3 The fragmentation to get the m/z = 43 peak is: H3C O Cl C CH CH3 O Cl C HC + H3C + CH3 m/z = 43 5