STEMI: INITIAL MANAGEMENT ORDER TEMPLATE (Referenced
advertisement

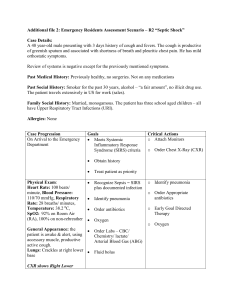
STEMI: INITIAL MANAGEMENT ORDER TEMPLATE (Referenced Version) This template lists initial elements, drugs, doses, and strategies that should be highly considered when creating admission order sets based upon recent clinical practice guidelines, medication package inserts, and emerging evidence. DEMOGRAPHICS Age ___________ years Weight __________ kg DIET ORDERS NPO Diabetic _________________ calorie ADA 2 gm sodium restricted/low cholesterol/low fat Other (specify) _____________________________ ACTIVITY ORDERS Bed rest NURSING1(pg e35) Continuous ECG monitoring Supplemental O2 to keep arterial saturation > 90% Nitroglycerin Protocol: PRN use of 0.4mg q5 min for chest pain MEDICATION ALLERGIES Specify: _______________________________________ Reaction (if known): ___________________________ DIAGNOSTIC STUDIES 12-lead ECG FOR RECURRENT CHEST PAIN 12-lead ECG IN AM (____/___/___ ___:___ AM) LABORATORY STUDIES ROUTINE LABS Chemistry panel: CBC: NOW DAILY IN AM NOW DAILY IN AM Fasting lipid panel: HbA1c: IN AM IN AM Calculate creatinine clearance (CrCl): _______ml/min CrCl ml/min = (140 - age) X weight (kg)/(serum creatinine X 72) multiply by 0.85 if female SELECT REPERFUSION STRATEGY Fibrinolytic Therapy: Within 12 hours of symptom onset and primary PCI cannot be achieved in <90 minutes of 1st medical contact OR who cannot be transferred to primary PCI center with anticipated time from 1st medical contact to balloon to be < 90 minutes1 FIBRINOLYTIC THERAPY ORDERS (Goal is Door to Needle <30 min) Primary PCI: Within 12 hours of symptom onset, with primary PCI facility available, with anticipated 1 st medical contact to balloon time < 90 minutes2 PRIMARY PCI ORDERS (Goal is Door to Balloon <90 min) Page 1 of 5 Medical management: Contraindications to reperfusion therapy. MEDICAL MANAGEMENT STRATEGY ORDERS MEDICATIONS FOR ALL STEMI PATIENTS Aspirin: Initial dose 162 mg to 325 mg chewed orally even if already taking aspirin,3 then daily dose of 81 mg to 162 mg orally4 if fibrinolytic or no reperfusion and 162 mg to 325 mg daily if primary PCI with stenting performed5 Initial: ___________ mg orally. Daily: ___________ mg orally. Clopidogrel: Fibrinolytic or No Reperfusion Patients: Initial Dose of 300 mg orally and maintenance dose of 75 mg.6 PCI Patients: Initial Dose of 300-600 mg orally and maintenance dose of 75 mg.7 Initial: ___________ mg orally. (No evidence for loading dose if age ≥75 year) Daily: ___________ mg orally. (Continue for 14 days and potentially to 1 year in fibrinolytic and no reperfusion patients8 and for 1 year in PCI and stent patients (may be 1 month if bare metal)9 ;hold if CABG is planned for at least 5-7 days)10 BETA BLOCKERS (Hold if signs of heart failure, evidence of low-output state, high risk for cardiogenic shock* or contraindications to beta blocker therapy**)11 Oral Beta-blocker: Drug: ____________________ _______ mg _____ times day IV Beta-Blockers (Optional; recommended if persistent ischemic symptoms, hypertension, or tachycardia and no signs of hemodynamic instability)12 Drug: ___________________ _____ mg IV for ____ doses every ___ minutes *Risk factors for shock (greater number of risk factors, the greater the risk): age > 70 years, SBP < 120 mmHg, sinus tachycardia with HR > 110 bpm or < 60 mmHg, or increased time since onset of symptoms.13 **Contraindications for beta-blockers: PR interval > 0.24 sec, second or third degree AV block, active asthma, or reactive airway disease.14 ACE-I or ARB: Drug: ____________________ _______ mg _____ times day (start 12 hours after admission). Statin: Drug: __________________________ ________ mg once daily (regardless of LDL) Stop NSAIDS (except aspirin) GI Prophylaxis (with history of GI bleeding) Drug: _________________________ ___________ mg _____________ times day FIBRINOLYTIC THERAPY ORDERS (Goal Door to Needle <30 min) Absolute Contraindications: Prior hemorrhagic CVA Any cerebrovascular events < 1 year Active internal bleeding Known intracranial neoplasm Suspected aortic dissection FIBRINOLYTIC THERAPY (Choose ONE): Streptokinase: OR Page 2 of 5 1.5 MU IV over 30-60 minutes16 Relative Contraindications: Blood pressure > 180/110 mm Hg Use of anticoagulants with INR > 2.0 Noncompressible vascular punctures Prolonged CPR (> 10 minutes) Prior gastrointestinal hemorrhage Pregnancy or Menstruation Trauma < 2 to 4 weeks prior Major Surgery <3 weeks prior15 Alteplase: Bolus: 15 mg IV Infusion: 0.75 mg/kg IV over 30 minutes (not to exceed 50 mg); then 0.5 mg/kg over the next 60 minutes (not to exceed 35 mg over the next 60 minutes)17 OR Reteplase: 10 U IV over 2 minutes, repeat after 30 minutes18 OR Tenecteplase: if if if if if weight weight weight weight weight < 60 kg, give 30 mg single IV bolus; 60-69 kg, give 35 mg single IV bolus; 70-79 kg, give 40 mg single IV bolus; 80-89 kg, give 45 mg single IV bolus; > 90 kg, give 50 mg single IV bolus. 19 ANTICOAGULANT THERAPY (Choose ONE with fibrinolytics): Unfractionated Heparin: Bolus: 60 U/kg IV (not to exceed 4000 U regardless of weight) Infusion: 12 U/kg/hr IV (not to exceed 1000U/hr regardless of weight) to goal PTT 1.5 to 2.0 times local reference standard; check PTT in 6 hours and adjust heparin as indicated Note: Not to be continued for > 48 hours unless otherwise indicated.20 OR Enoxaparin*: Bolus: 30 mg IV (no IV bolus if age >75 years) Maintenance: 1mg/kg subcutaneously every 12 hours, first dose 15 minutes after bolus (if age >75 years give 0.75 mg/kg every 12 hours with no bolus; if CrCl <30 mL/min, give 1 mg/kg every 24 hours after bolus)21 OR Fondaparinux*: 2.5 mg IV initial dose, then 2.5mg subcutaneously daily thereafter (avoid if CrCl ≤30 mL/min)22 *Anticoagulant therapy for at least 48 hours, and preferably for duration of hospitalization, up to 8 days. PRIMARY PCI STRATEGY ORDERS (Goal Door to Balloon <90 min) GLYCOPROTEIN IIB/IIIA THERAPY (Choose one (Class IIa) or use clopidogrel as above) Abciximab Loading: 0.25 mcg/kg IV bolus Infusion 0.125 mcg/kg/min IV for 12 hours23 OR Eptifibatide: Loading: 180 mcg/kg IV bolus, repeat bolus after 10 minutes Infusion: 2.0 mcg/kg/min IV for 12-18 hours (Reduce to 1.0 mcg/kg/min if CrCl <50 mL/min)24 OR Tirofiban: Loading: 0.4 mcg/kg/min for 30 minutes IV (Reduce to 0.2 mcg/kg/min for CrCl ≤30 mL/min) Infusion: 0.1 mcg/kg/min IV for 12-18 hours (Reduce to 0.05 mcg/kg/min if CrCl ≤30mL/min)6 *Can be started prior to or in cardiac catheterization lab. As supplement to clopidogrel. ANTICOAGULANT THERAPY (Choose one): Unfractionated Heparin: Bolus: 60 U/kg not to exceed 4000 U Infusion: 12 U/kg/hr not to exceed 1000 U/hr to goal PTT 1.5 to 2.0 times local reference standard (target ACT in catheterization lab: 200-250 sec if concomitant GP IIb-IIIa therapy, 250-300 sec if no concomitant GP IIb-IIIa therapy; re-bolus with heparin to achieve target ACT if measured values below recommended ranges)25 OR Page 3 of 5 Enoxaparin: Bolus: 30 mg IV (no IV bolus if age >75 years) Maintenance: 1mg/kg subcutaneously every 12 hours, first dose 15 minutes after bolus (if age >75 years give 0.75 mg/kg every 12 hours with no bolus; if CrCl <30 mL/min, give 1 mg/kg every 24 hours after bolus)26 If last subcutaneous dose less than 8 hours prior to PCI, no additional enoxaparin required; if last subcutaneous dose 8-12 hours earlier or never given, intravenous dose of 0.3 mg/kg should be given. Note: Discontinue after completion of the PCI procedure, unless continued anticoagulation indicated MEDICAL MANAGEMENT STRATEGY ORDERS (NO REPERFUSION) ANTICOAGULANT THERAPY (Choose one): Unfractionated Heparin: Bolus: 60 U/kg IV (not to exceed 4000 U regardless of weight) Infusion: 12 U/kg/hr IV (not to exceed 1000U/hr regardless of weight) to goal PTT 1.5 to 2.0 times local reference standard; check PTT in 6 hours and adjust heparin as indicated Note: Not to be continued for > 48 hours unless otherwise indicated.27 OR Enoxaparin*: Bolus: 30 mg IV (no IV bolus if age >75 years) Maintenance: 1 mg/kg subcutaneously every 12 hours, first dose 30 minutes after bolus (if age > 75 years, give 0.75 mg/kg every 12 hours with no bolus; if CrCl <30 mL/min, give 1 mg/kg every 24 hours after bolus)28 OR Fondaparinux*: 2.5 mg IV initial dose, then 2.5mg subcutaneously daily thereafter (avoid if CrCl ≤30 mL/min)29 *Anticoagulant therapy for at least 48 hours, and preferably for duration of hospitalization, up to 8 days in patients with no contraindications for anticoagulation. Copyright © 2008 by the American College of Cardiology. This content is owned by the ACC. A person accessing it may print the material and use it for his or her personal, institutional, non-commercial reference. Other terms and conditions for use may apply, particularly for commercial purposes. For more details, please contact the ACC in writing. This material is intended for reference and does not represent ACC policy. This content is not endorsed by the ACC. If any modifications are made to the content or layout of this material, all representation of the material as derived from ACC or NCDR should be removed. Page 4 of 5 Endnote: 1 Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210-247. (p217). 2 Antman EM, Hand M, Armstrong PW, et al. 2007, p 217. 3 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211. (p36). Antman EM, Anbe DT, Armstrong PW, et al.2004, e73. Antman EM, Hand M, Armstrong PW, et al. 2007, p 235. 6 Antman EM, Hand M, Armstrong PW, et al. 2007, p 230. 7 King SB, 3rd, Smith SC, Jr., Hirshfeld JW, Jr., et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172-209. 8 Antman EM, Hand M, Armstrong PW, et al. 2007, p 237 9 Antman EM, Hand M, Armstrong PW, et al. 2007, p 217. 10 Antman EM, Hand M, Armstrong PW, et al. 2007, p 237 11 Antman EM, Hand M, Armstrong PW, et al. 2007, p 215 12 Antman EM, Hand M, Armstrong PW, et al. 2007, p 215 13 Antman EM, Hand M, Armstrong PW, et al. 2007, p 215 14 Antman EM, Hand M, Armstrong PW, et al. 2007, p 215 15 Antman EM, Anbe DT, Armstrong PW, et al.2004, e45. 16 Antman EM, Anbe DT, Armstrong PW, et al.2004, e53. 17 Antman EM, Anbe DT, Armstrong PW, et al.2004, e53. 18 Antman EM, Anbe DT, Armstrong PW, et al.2004, e53. 19 Antman EM, Anbe DT, Armstrong PW, et al.2004, e53. 20 Antman EM, Anbe DT, Armstrong PW, et al.2004, e53. 21 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224. 22 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224. 23 Reopro (abciximab) Package Insert. Available at: http://www.centocor.com/centocor/assets/reopro.pdf. Accessed June 12, 2008. 24 Integrilin (eptifibatide) Package Insert. Available at: http://www.integrilin.com/pdf/Integrilin_Prescribing_Information.pdf. Accessed June 12, 2008. 25 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224. 26 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224 27 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224 28 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224 29 Antman EM, Hand M, Armstrong PW, et al. 2007, p 224 4 5 Page 5 of 5