Aigars Ekers. ENERGY TRANSFER AND IONISATION
advertisement

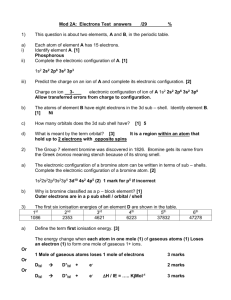
2. COLLISIONAL IONISATION PROCESSES Besides the energy transfer in collisions of excited atoms and / or molecules, also ionisation is possible. Three types of processes can be distinguished: A* + B* (i) A+ + B, (ion pair formation) (2.1a) (ii) A+ + B + e, (Penning ionisation) (2.1b) (iii) AB+ + e. (associative ionisation) (2.1c) Ion pair formation takes place thanks to non-adiabatic transitions between covalent (AB**) and ionic (A+B) terms at large internuclear distances. Penning (PI) and associative (AI) ionisation result from autoionisation of quasimolecular continuum states (AB**) at small internuclear distances. AI possess the smallest threshold energy among the other ionisation processes, and it may prove itself as an important mechanism in cluster formation. Recently, a hypothesis was proposed that dissociative recombination of trimer ions might be responsible for the violet excimer type emission on the 2 3 g a 3 u transition in Na2 molecule [25,67,68]: Na 3 + e Na2( 23 g ) + Na, where trimer ions could be formed in some of the molecule-atom AI processes, e.g., Na2( C 1 u ) + Na(3S) Na 3 + e, Na2( A1 u ) + Na(3P) Na 3 + e. (2.2) The purpose of the present work is to prove the formation of trimer ions in collisions of excited atoms and molecules, and to clarify the influence of initial vibrational excitation of Na2 molecule on the efficiency of AI. 2.1. THEORETICAL INTERPRETATION OF ASSOCIATIVE IONISATION For the sake of simplicity, let us consider a collision of two excited atoms, A* and B*, following [21,69,70]. As the atoms approach each other, a quasimolecular system is formed which may rich an autoionising state and emit an electron. AI can take place when the total excitation energy is larger than the ionisation potential of an atom (Fig. 2.1a), as well as when it is smaller (Fig. 2.1b). Usually one works in the BornOppenheimer approximation, which is valid at small (thermal) collision energies of the particles. The dynamics of the process can be fully described by three local functions: molecular potentials U R and U R , which correspond to the covalent entrance and 20 Fig. 2.1. Schematic illustration of covalent [ U R ] and ionic [ U R ] potential curves. E0 is the initial kinetic energy of the collision, but E(R) is the kinetic energy of nuclei at internuclear distance R. According to the Franck-Condon principle, E(R) does not change during the electronic transition, i.e., autoionisation results in a transition to the so-called difference term (broken line). (a) The sum of potential energies of A and B is larger than the ionisation potential of atom B. Depending on the internuclear distance at which the transition happens, either Penning (R>RAI) or associative (R0RRAI) ionisation will take place. (b) The sum of potential energies of A and B is smaller than the ionisation potential of atom B. Autoionisation is possible only at internuclear distances R0RRc. In the displayed case, U E0 U , therefore only the associative ionisation takes place. ionic exit channels, and the autoionisation width of the covalent state (R)2. Molecule in an autoionising state U R may spontaneously emit an electron to go over to the ionic state U R . Franck-Condon principle anticipates that this electronic transition does not change the kinetic energy and momentum of the relative motion of the nuclei. The total energy of the system after collision will be W R E0 U U R U R , where energy R U R U R is carried away by the free electron. Depending on if W(R) will be smaller or larger than U+(), in the case shown in Fig. 2.1a either associative (at R0 R RAI) or Penning (at R > RAI) ionisation will take place. In the case shown in Fig. 2.1b only the associative ionisation is possible. At a given initial angular momentum of nuclei, l , ionisation probability can be expressed as [70] 2 R is the lifetime of the system of colliding particles with respect to autoionisation, but (R) is determined as R 2g Ĥ 2 , where and are the wavefunctions of the inital and final states, Ĥ is the hamiltonian of the system, and g is the density of continuum states. 21 R Pl Pl R dR 1 exp 2 dR , Rl v l R Rl (2.3) where Pl(R) accounts for the dependence of ionisation probability on R; Rl is the ldependent turning point on U R , but the radial velocity of the nuclei, vl, can be determined from the initial kinetic energy E0 as vl 2 2l 2 E0 U U R , 2R 2 (2.4) where is the reduced mass of the system A-B. The total ionisation cross section is obtained from (2.3) by summing it over different values of l: tot E0 2 2l 1P E . l 0 (2.5) l Since the main contribution to the cross section is due to large l values, the discrete angular momenta can be replaced by the relation l 1 2 , where is the impact parameter, and 2E0 . Transforming the sum (2.5) into integral over , one obtains: tot E0 2 P d , (2.6) 0 where P() is calculated from (2.3) and (2.4) with l replaced in the above described way. In the case of AI, the upper limit of integral (2.6) should be replaced by max, which is determined by the maximal internuclear distance RAI [Fig. 2.1(a)], or Rc [Fig. 2.1(b)], at which the associative ionisation is still possible. In order to compare with the experimental cross sections, (2.6) should be averaged over the corresponding distributions f(E0) of energy E0 (i.e., the collision velocity v 0 2E0 ): tot tot E 0 f E 0 dE 0 . Distribution functions for different experimental conditions (single and crossed beams, thermal cells) are considered in [1,38]. Detailed theoretical calculations for particular reactions require precise information on behaviour of the molecular terms at small internuclear distances, which is by itself a complicated task of quantum chemistry. Therefore, in most cases qualitative molecular potentials are used (see, e.g., [71]), which limits the accuracy of 22 calculations. The calculations are complicated also by a fact that it is necessary to take into consideration the energy-transfer-type processes described in part 1, which influence the probability that the system will survive on the initial tem U R until the beginning of autoionisation. Moreover, at small internuclear distances one has to account for interactions with Rydberg terms, which not only diminish the population of U R , but may also give a contribution to the ionisation (see, e.g., [72]). Until now calculations based on accurate molecular terms have been possible only for the well-known Na(3p) + Na(3p) AI reaction [20,72]. As about the AI in collisions of molecules and atoms, it has not been treated theoretically until now. 2.2. MOLECULE-ATOM ASSOCIATIVE IONISATION Contrary to the associative ionisation in atom-atom collisions, only few qualitative experiments have been conducted until recently on molecule-atom AI, with either rare gas atoms or N2 molecules as one of the collision partners [23,24,73,74]. Molecule-atom AI in alkalis was observed for the first time recently in collisions of electronically excited molecules with ground state atoms [75]: Na2( 21 g ) + Na Na 3 . (2.7) The authors of [75] used the process (2.7) as an ion source in an ionisation detector diode, and attempts to study the very AI process were not reported. In another study [26] AI in collisions of ground state molecules and excited atoms was considered: Na2( X 1 g ) + Na(4D) Na 3 + e; (2.8) Na2( X 1 g ) + Na(5S) Na 3 + e. (2.9) These experiments were performed in an effusive Na / Na2 beam, using time-of-flight (TOF) mass spectrometry to distinguish among different ionic products. The cross sections of processes (2.8) and (2.9) were determined relative to those of the atom-atom AI reactions [76]: Na(3S) + Na(4D) Na 2 + e, (2.10) Na(3S) + Na(5S) Na 2 + e. (2.11) The experiments showed that the cross sections of molecule-atom AI exceed those of the corresponding atomic reactions (2.10) and (2.11) two times in the case of the 4D 5/2 state, and 10 times in the case of the 5S1/2 state. Except the above mentioned processes, no more data are available in the literature on the molecule-atom AI. 23