Nuclear pharmacy lecture 1
advertisement

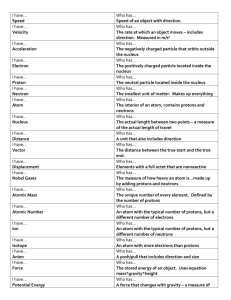
Nuclear pharmacy lecture 1 Introduction All substances are made of atoms. These have electrons (e) around the outside (negatively charged), and nucleus in the middle. The nucleus consists of protons (positively charged) and neutrons (neutral), collectively called nucleons. Protons are positively charged with a mass 1800 times that of electrons. Neutrons have the same mass like protons but carry no charge. The atom All matter is made up of elements (e.g. carbon, hydrogen, etc.). The smallest part of an element is called an atom. Atom of different elements contains different numbers of protons. The mass of an atom is almost entirely due to the number of protons and neutrons. The Atom The atom consists of two parts: 1. The nucleus which contains: Protons Neutrons 2. Orbiting electrons For an atom of a given element, the number of electrons moving around the nucleus equals the number of protons, balancing the electrical charge of the nucleus. 1 The electron configuration of the atom determines the chemical properties of an element. The nuclear structure characterizes the stability and radioactive decay of the nucleus of an atom. The atom has a radius of approximately 10-8 cm, with the nucleus having a radius of 10-12 cm. → The mass M of a nucleus is always less than the combined masses of the nucleons A in the nucleus. → This difference in mass (M-A) is termed the ‟mass defect”, which has been utilized as energy in binding all the nucleons in the nucleus. → This energy is the ‟ binding energy” of the nucleus and needs to be supplied to separate all nucleons completely from each other. → The binding energy of an individual nucleon has a definite value depending upon the shell it occupies, it is approximately equal to the total binding energy divided by the number of nucleons. → This energy is about 6 to 9 MeV and has to be supplied to remove a single nucleon from the nucleus. 2 Structure of the nucleus Mass number = number of protons + number of neutrons A Z Elemental atom X N Neutron number Atomic number = number of protons Electronic structure of the Atom The electrons are located in discrete energy orbits or shells around the nucleus (Bohr atomic theory). These energy shells, referred to as the K shell, L shell, N shell, etc., are stationary and arranged in order of increasing energy. Each orbit has a limited capacity to hold only a definite number of electrons. That K contains 2 electrons, L contains 8 electrons. 3 ► Each shell is designated by quantum number n, called the principal quantum number. ► Each energy shell is subdivided into subshells or orbitals, which are designated as s, p, d, f, etc. (azimuthal quantum numbers, l). l = 0,1,2……etc. ► The electron will enter the orbital of the lowest energy first and the next higher energy orbital is then filled. For example: 11Na 1s2 2s2 2p6 3s1 BOHR ATOM electrons: n = principal quantum number 1 n=3 2 Nucleus: Z = # protons = 1 for hydrogen to 94 for plutonium N = # neutrons Atomic mass A ≈ Z + N n labels shells; shells are composed of sub-shells: s, p, d, f, … 4 The periodic table The periodic table is a chemistry reference. Elements are arranged left to right and top to bottom in order of increasing atomic number. This order usually coincides with increasing atomic mass. The different rows of elements are called periods. Elements of each row have the same number of energy levels (shells) but dissimilar chemical properties. The period number of an element signifies the highest energy level an electron in that element occupies. The different columns of elements are called groups. Elements in the same group have similar chemical properties. Describe the Periodic Table Atomic Mass: The atomic mass is the average mass of an element in atomic mass units ("amu"). 5 Models for the arrangement of nucleons in a nucleus 1. Bohr liquid drop theory: The nucleus is assumed to be spherical and composed of closely packed nucleons, and particle emission by the nucleus resembles evaporation of molecules from a liquid drop. 2. In the shell model, nucleons are arranged in discrete energy shells similar to the electron shells of the atom in the Bohr atomic theory. Nuclei containing 2, 8, 20, 50, 82 or 126 protons or neutrons are very stable and the nucleon numbers are called magic numbers. 3. Nuclei are less stable if they contain an odd number of protons or neutrons, whereas nuclei with even numbers of protons and neutrons are more stable. 6 The stability of the atom depends on the neutrons to protons ratio (N/Z) ratio in the nucleus. This ratio equals 1 in the stable nuclei with a low atomic number, such as 126C, 168O and 147N, and the ratio increases with increasing the atomic number of the nucleus. For example, this ratio is 1.40 for 12753I. Electrons distribution Valence electrons: ●● Electrons in the highest (outer) electron shell. ●● Have most contact with other atoms. ●● They are distributed as shared or bond pairs and unshared or lone pairs. Octet Rule: ● All chemical bond formation is governed by the octet rule that the electronic structure of each atom in a chemical bond containing 8 electrons. ● An octet in the outer shell makes atoms stable. ● Electrons are lost, gained or shared to form an octet. ● Metals lose electrons to match the number of valence electrons of their nearest noble gas e.g. He [2], neon [2,8] 7 Octet Rule = atoms tend to gain, lose or share electrons so as to have 8 electrons Chemical bonds: an attempt to fill electron shells 1. Ionic bonds 2. Covalent bonds 3. Covalent coordinate 8 1. IONIC BOND - bond formed between two ions by the transfer of electrons. - Ionic compounds result when metals react with nonmetals. - Produce charged ions all states. Conductors and have high melting point. Examples; NaCl, CaCl2 Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. 2. COVALENT BOND - Bond formed by the sharing of electrons. It means that each of the atoms participating in bond formation contributes one electron to the bond. - The shared electrons are localized in the region between the two atoms and unlike to the ionic bonds, do not belong exclusively to one atom alone. - Stable non-ionizing molecules, they are not conductors at any state. Examples; O2, CO2, C2H6, H2O 9 Covalent bonds: Two atoms share one or more pairs of outer-shell electrons. A. Non-Polar covalent bond When electrons are shared equally, for example H2 or Cl2 Oxygen Atom Oxygen Atom Oxygen Molecule (O2) 10 B. Polar covalent bond when electrons are shared but shared unequally, for example Hcl, H2O - water is a polar molecule because oxygen is more electronegative than hydrogen, and therefore electrons are pulled closer to oxygen. 3. Coordinate covalent bond The pair of electrons required for bond formation is donated by only one atom to another that can accommodate two electrons in octet formation. Nitrogen and oxygen atoms have donated their lone pair of electrons to a hydrogen bond. 11 In a coordination complex: an arrow is used to show the bonding interaction of an electron pair from a neutral ligand to an acceptor central atom. [Co(H2O)6]2+ + 6 NH3 -----> [Co(NH3)6]2+ + 6 H2O Ligand or complexing agent 2+ NH3 H3N NH3 Co NH3 H3N Metal ion NH3 Donation of unshared pair of electrons Ethylene Diamine Tetraacetic Acid (EDTA) Chelating agent ● Single ligand molecule can possess more than one donor atom and can donate more than one pair of electrons in the complex. ● In such cases, more than one coordinate covalent bond is formed in the complex. The mechanism of bond formation is called chelation. 12 13