Expand Long Template PCR
advertisement
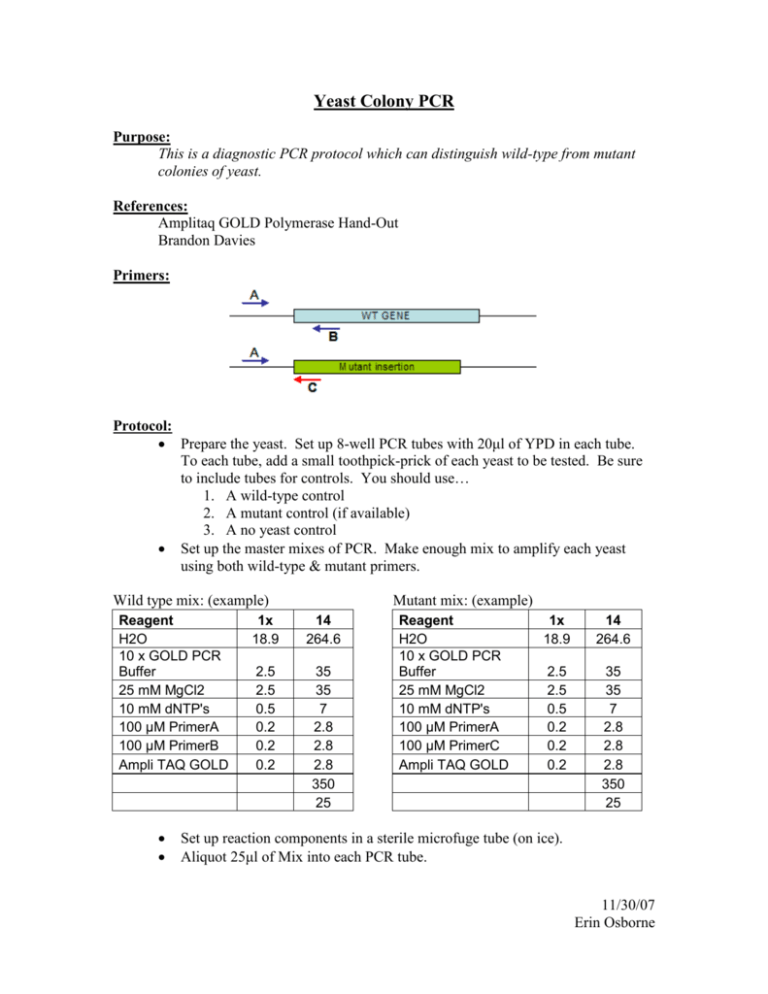
Yeast Colony PCR Purpose: This is a diagnostic PCR protocol which can distinguish wild-type from mutant colonies of yeast. References: Amplitaq GOLD Polymerase Hand-Out Brandon Davies Primers: Protocol: Prepare the yeast. Set up 8-well PCR tubes with 20μl of YPD in each tube. To each tube, add a small toothpick-prick of each yeast to be tested. Be sure to include tubes for controls. You should use… 1. A wild-type control 2. A mutant control (if available) 3. A no yeast control Set up the master mixes of PCR. Make enough mix to amplify each yeast using both wild-type & mutant primers. Wild type mix: (example) Reagent H2O 10 x GOLD PCR Buffer 25 mM MgCl2 10 mM dNTP's 100 μM PrimerA 100 μM PrimerB Ampli TAQ GOLD Mutant mix: (example) 1x 18.9 14 264.6 2.5 2.5 0.5 0.2 0.2 0.2 35 35 7 2.8 2.8 2.8 350 25 Reagent H2O 10 x GOLD PCR Buffer 25 mM MgCl2 10 mM dNTP's 100 μM PrimerA 100 μM PrimerC Ampli TAQ GOLD 1x 18.9 14 264.6 2.5 2.5 0.5 0.2 0.2 0.2 35 35 7 2.8 2.8 2.8 350 25 Set up reaction components in a sterile microfuge tube (on ice). Aliquot 25μl of Mix into each PCR tube. 11/30/07 Erin Osborne Using a Multi-Channel Pipet, add 1μl of yeast cultures to each PCR tube. Cap tubes. Run the Thermocycle Profile 1. 94C 13:00 2. 94C 1:00 3. 50C 0:30 4. 72C 2:00*** 5. Goto Step2 34x 6. 72C 10:00 7. 4C for ever 8. END *** This time changes as your expected product length changes. Elongation time = 1min/1kb fragment + 1 min Add tubes to the thermocycler. To verify the products, run 10μl of each reaction on a 1 – 1.5% agarose gel + EtBr. 11/30/07 Erin Osborne











