Expand Long Template PCR
advertisement
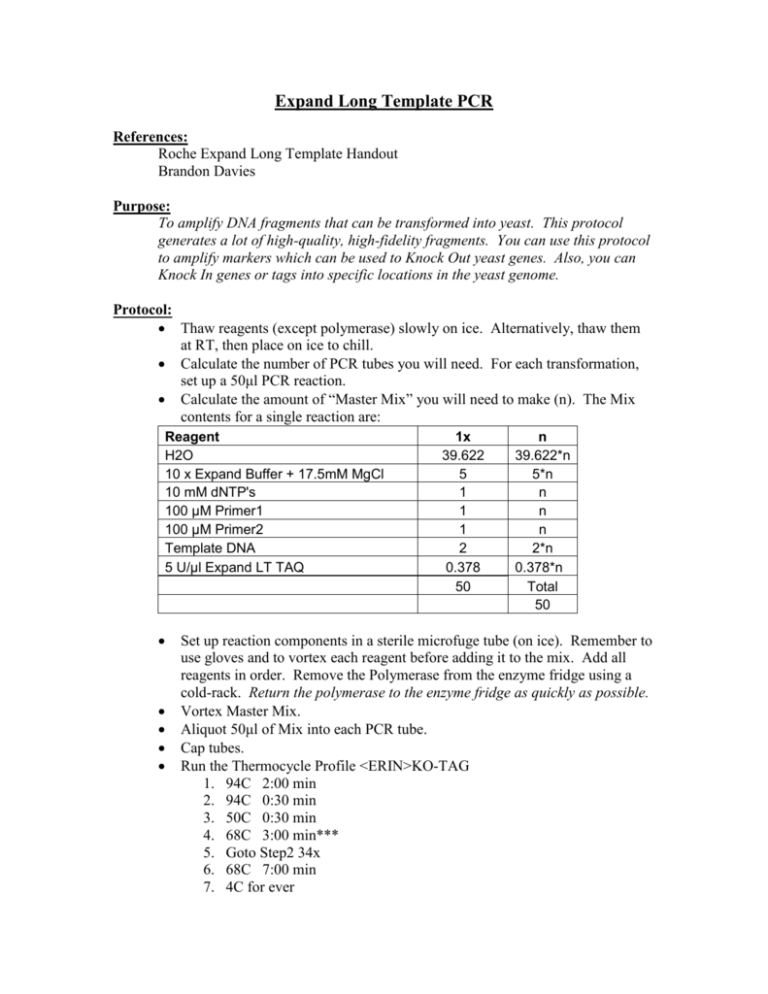
Expand Long Template PCR References: Roche Expand Long Template Handout Brandon Davies Purpose: To amplify DNA fragments that can be transformed into yeast. This protocol generates a lot of high-quality, high-fidelity fragments. You can use this protocol to amplify markers which can be used to Knock Out yeast genes. Also, you can Knock In genes or tags into specific locations in the yeast genome. Protocol: Thaw reagents (except polymerase) slowly on ice. Alternatively, thaw them at RT, then place on ice to chill. Calculate the number of PCR tubes you will need. For each transformation, set up a 50μl PCR reaction. Calculate the amount of “Master Mix” you will need to make (n). The Mix contents for a single reaction are: Reagent H2O 10 x Expand Buffer + 17.5mM MgCl 10 mM dNTP's 100 μM Primer1 100 μM Primer2 Template DNA 5 U/μl Expand LT TAQ 1x 39.622 5 1 1 1 2 0.378 50 n 39.622*n 5*n n n n 2*n 0.378*n Total 50 Set up reaction components in a sterile microfuge tube (on ice). Remember to use gloves and to vortex each reagent before adding it to the mix. Add all reagents in order. Remove the Polymerase from the enzyme fridge using a cold-rack. Return the polymerase to the enzyme fridge as quickly as possible. Vortex Master Mix. Aliquot 50μl of Mix into each PCR tube. Cap tubes. Run the Thermocycle Profile <ERIN>KO-TAG 1. 94C 2:00 min 2. 94C 0:30 min 3. 50C 0:30 min 4. 68C 3:00 min*** 5. Goto Step2 34x 6. 68C 7:00 min 7. 4C for ever 8. END *** This time changes as your expected product length changes. Elongation time = 1min/1kb fragment + 1 min Add tubes to the thermocycler. To verify the products, run 3μl of each reaction on a 1 – 1.5% agarose gel + EtBr. Variations on a Theme If you have a lot of different fragments to generate, you can make a general master mix, aliquot it, and then add the individual primers to the tubes one-ata-time. Troubleshooting No band? Here are some possibilities: 1. Check that the primer is correct. 2. Check that the template good. 3. Try a lower Annealling temp (lower than 50C) 4. Try a higher magnesium concentration 5. Try a longer elongation time. Too many bands? 1. Try a higher annealing temp (higher than 50C) 2. Try a lower magnesium concentration Primer dimer? 1. re-design primers without overlapping regions. 2. Lower the amount of template DNA.











