Chemistry I-Honors - smhs
advertisement

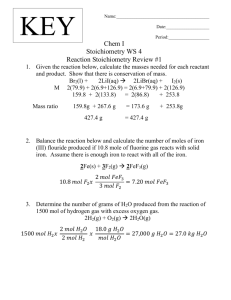
Chemistry I-Honors Intro Stoichiometry Problem Set Solution Set For all of these reactions, you will need to write out the balanced equation. 1. 40.0 grams of magnesium metal is reacted with an excess of an aqueous solution of phosphoric acid. How many liters of hydrogen gas are released, assuming STP conditions? 3 Mg(s) + 40.0 g Mg 2 H3PO4(aq) Mg3(PO4)2(s) + 3 H2(g) 1 mol Mg 3 mol H2 22.4 L x -------------- x ------------- x ------------ = 36.9 L H2 24.3 g Mg 3 mol Mg 1 mol H2 Consider the following information for the next three questions: 35.3 grams of solid cesium hydroxide is reacted with an excess of 12.0 M hydrochloric acid. 1 CsOH(s) 2. + 1 HCl(aq) 1 CsCl(aq) + 1 H2O How many grams of water should be produced theoretically? 35.3 g CsOH 1 mol CsOH 1 mol water 18.0 g water x ---------------- x ----------------- x ---------------- = 4.24 g H2O 149.9 g CsOH 1 mol CsOH 1 mol H2O 3. The other product in the above reaction is cesium chloride. If the solution is heated until only the salt remains behind, how many grams of cesium chloride could be recovered theoretically? 35.3 g CsOH 1 mol CsOH 1 mol CsCl 168.4 g CsCl x ---------------- x ----------------- x ---------------- = 39.7 g CsCl 149.9 g CsOH 1 mol CsOH 1 mol CsCl 4. If 40.0 grams of cesium chloride are actually collected, what is the percent yield in this experiment? How would this be possible? What would be a likely source of error? (40.0 g / 39.7 g) x 100 = 101% yield Probably all of the water was not driven off. 5. How many liters of ammonia gas can be produced if 60.0 liters of hydrogen gas are reacted with an excess of nitrogen gas at STP conditions? N2(g) + 3 H2(g) 2 NH3(g) 60.0 L H2 x (2 mol ammonia / 3 mol hydrogen ) = 40.0 L NH3 -26. How many moles of silver nitrate are needed to react completely with an aqueous solution of auric chloride in order to have 80.0 grams of silver chloride precipitate out? 3 AgNO3(aq) + AuCl3(aq) Au(NO3)3(aq) 1 mol AgCl 3 mol AgNO3 80.0 g AgCl x ----------------- x -------------------143.4 g AgCl 3 mol AgCl 7. + 3 AgCl(ppt) = 0.558 mol AgNO3 How many grams of sodium chlorate need to be heated (decomposed) in order to yield 25.0 liters of oxygen gas at STP conditions? 2 NaClO3(s) 2 NaCl(s) + 3 O2(g) 1 mol O2 2 mol NaClO3 106.5 g NaClO3 25.0 L O2 x ------------- x ------------------ x ------------------- = 79.2 g NaClO3 22.4 L O2 3 mol O2 1 mol NaClO3 8. If 2.00 liters of a 4.00 M aqueous solution of zinc nitrate are reacted with an excess of an aqueous solution of sodium sulfide, how many grams of the zinc sulfide should precipitate theoretically? Zn(NO3)2(aq) 2.00 L 9. 2 NaNO3(aq) + ZnS(ppt) 4.00 mol Zn(NO3)2 1 mol ZnS 97.5 g ZnS x ---------------------- x ------------------- x -------------- = 780. g ZnS 1 L soln. 1 mol Zn(NO3)2 1 mol ZnS What volume of a 6.00 M solution of sulfuric acid must be reacted with an excess of solid lithium hydroxide in order to yield 50.0 grams of water as a product of this neutralization reaction? 2 LiOH(s) + 50.0 g H2O 10. + Na2S(aq) H2SO4(aq) Li2SO4(aq) + 2 H2O(liq) 1 mol H2O 1 mol acid 1 L soln. x -------------- x --------------- x -----------------18.0 g H2O 2 mol H2O 6.00 mol acid = 0.231 L acid When 70.0 grams of solid calcium carbonate are heated, how many liters of carbon dioxide can be recovered theoretically, if the reaction is run at STP conditions? If 13.8 liters of CO2 are collected, what is the percent yield of this experiment? 1 CaCO3(s) 70.0 g chalk 1 CaO(s) + 1 CO2(g) [CaCO3 is chalk] 1 mol chalk 1 mol CO2 22.4 L CO2 x ----------------- x -------------- x ---------------100.1 g chalk 1 mol chalk 1 mol CO2 % yield = (13.8 / 15.7) x 100 = 87.9% = 15.7 L CO2