Balancing Equations
advertisement
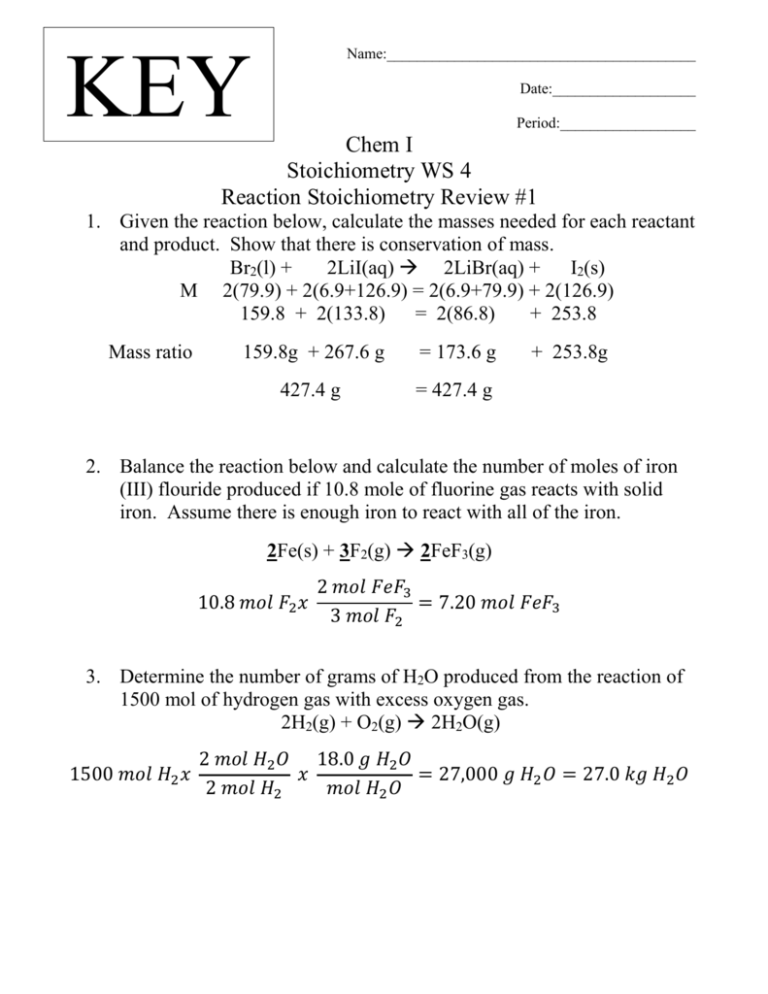
Name:_________________________________________ KEY Date:___________________ Period:__________________ Chem I Stoichiometry WS 4 Reaction Stoichiometry Review #1 1. Given the reaction below, calculate the masses needed for each reactant and product. Show that there is conservation of mass. Br2(l) + 2LiI(aq) 2LiBr(aq) + I2(s) M 2(79.9) + 2(6.9+126.9) = 2(6.9+79.9) + 2(126.9) 159.8 + 2(133.8) = 2(86.8) + 253.8 Mass ratio 159.8g + 267.6 g = 173.6 g 427.4 g = 427.4 g + 253.8g 2. Balance the reaction below and calculate the number of moles of iron (III) flouride produced if 10.8 mole of fluorine gas reacts with solid iron. Assume there is enough iron to react with all of the iron. 2Fe(s) + 3F2(g) 2FeF3(g) 10.8 𝑚𝑜𝑙 𝐹2 𝑥 2 𝑚𝑜𝑙 𝐹𝑒𝐹3 = 7.20 𝑚𝑜𝑙 𝐹𝑒𝐹3 3 𝑚𝑜𝑙 𝐹2 3. Determine the number of grams of H2O produced from the reaction of 1500 mol of hydrogen gas with excess oxygen gas. 2H2(g) + O2(g) 2H2O(g) 1500 𝑚𝑜𝑙 𝐻2 𝑥 2 𝑚𝑜𝑙 𝐻2 𝑂 18.0 𝑔 𝐻2 𝑂 𝑥 = 27,000 𝑔 𝐻2 𝑂 = 27.0 𝑘𝑔 𝐻2 𝑂 2 𝑚𝑜𝑙 𝐻2 𝑚𝑜𝑙 𝐻2 𝑂 4. Aluminum carbonate decomposes into aluminum oxide and carbon dioxide. Determine the volume of carbon dioxide, in liters, produced at 1.00 atm of pressure and 25°C if 2.35 g of alumium carbonate is decomposed. __Al2(CO3)3(s) __Al2O3(s) + 3CO2(g) Al: 2 x 27.0 = 54.0 g Al C: 3 x 12.0 = 36.0 g C O: 9 x 16.0 =144.0 g O 234.0 g/mol 1 𝑚𝑜𝑙 𝐴𝑙2 (𝐶𝑂3 )3 2.35 𝑔 𝐴𝑙2 (𝐶𝑂3 )3 𝑥 = 0.0100 𝑚𝑜𝑙 𝐴𝑙2 (𝐶𝑂3 )3 234.0 𝑔 𝐴𝑙2 (𝐶𝑂3 )3 3 𝑚𝑜𝑙 𝐶𝑂2 0.0100 𝑚𝑜𝑙 𝐴𝑙2 (𝐶𝑂3 )3 𝑥 = 0.0300 𝑚𝑜𝑙 𝐶𝑂2 1 𝑚𝑜𝑙 𝐴𝑙2 (𝐶𝑂3 )3 𝐿 ∗ 𝑎𝑡𝑚 (0.0300 𝑚𝑜𝑙 𝐶𝑂2 ) (0.0821 ) (298 𝐾 ) 𝑛𝑅𝑇 𝑚𝑜𝑙 ∗ 𝐾 𝑉= = 𝑃 1.00 𝑎𝑡𝑚 = 0.737 𝐿 𝐶𝑂2 (Note: what is volume of CO2 @ STP? 0.0300 x 22.4 L/mol = 672 mL) 5. Balance the reaction below and determine volume ratio of carbon monoxide to carbon dioxide. If 42.7 g of CO is reacted completely at STP, what mass in grams and volume in liters of nitrogen gas will be produced? 2CO(g) + 2NO(g) __N2(g) + _2CO2(g) M 28.0 28.0 Mass ratio 56.0 28.0 Volume ratio is 2CO: 2 CO2 or 1:1 42.7 g CO x 28.0g N2/56.0g CO = 21.4 g N2 21.4 g N2 x 1 mol N2 28.0 g N2 x 22.4 L mol gas = 17.1 L N2