The Carbonyl Compounds Aldehydes and Ketones
advertisement

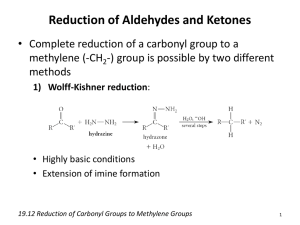
The Carbonyl Compounds Aldehydes and Ketones Aldehydes and Ketones both have the general formula, CnH2nO and both contain the carbonyl group, Aldehydes have an alkyl (or aryl) group and a hydrogen atom attached to the carbonyl carbon, whereas ketones have two alkyl (or aryl) groups attached to the carbonyl carbon atom. Methanal, the first aldehyde, has all of its atoms lying in the same plane. All other aldehydes and ketones have the three atoms around the carbonyl carbon lying in one plane. ALDEHYDES - at least one hydrogen atom attached to the carbonyl carbon atom KETONES - 2 carbon atoms attached Some examples of aldehydes and ketones are shown below : - Nomenclature Aldehydes • look for the longest chain of C atoms containing the carbonyl group equivalent alkane name and add ‘AL’ • remove ‘E’ from the • substituents are numbered based on the C with the O being number 1. Ketones • look for the longest chain of C atoms containing the carbonyl group equivalent alkane name and add ‘ONE’. • remove ‘E’ from the • if necessary, the position of the C=O is given (lower number counting from one end) • substituents are numbered based on the number allocated to the C in the C=O 1 Distinguishing between Aldehydes and Ketones Most of the reactions of Aldehydes and Ketones are similar and are due to the carbonyl functional group. They differ in their ability to be oxidised and this is the reaction that is used to distinguish them. Aldehydes are easily oxidised to carboxylic acids but ketones are difficult to oxidise. This is because aldehydes have a hydrogen atom attached to the carbonyl carbon atom but ketones do not. The oxidising agent used to distinguish them must be mild because powerful oxidising agents can oxidise ketones by rupturing the carbon – carbon bond. Mild oxidising agents will not affect ketones but will affect aldehydes. Tollens reagent is a solution of the complex ion [Ag(NH3)2] + , and is made by adding aqueous ammonia to a solution of silver nitrate. A brown precipitate of silver oxide appears which redissolves in excess ammonia. (Complex ions are often quite soluble) 2 Ag + + 2 NH4 OH Ag2 O Ag2 O + 4 NH4 OH 2 [Ag(NH3)2] + + H2 O + + 2 OH 2 NH4+ + 3 H 2O The Ag+ in the [Ag(NH3)2] + complex ion is very easily reduced to silver metal and so when a few drops of an aldehyde are added to a few cm3 of tollens reagent in a perfectly clean test tube and carefully warmed, metallic silver is deposited on the walls of the test tube as a mirror. Ketones have no effect. Ag+ + e Ag A safety aspect of this particular reaction is that the product mixture must be disposed of as soon after the reaction as possible because Tollens Reagent deteriorates rapidly forming an explosive complex. Fehling’s Solution or Benedict’s Solution is a solution containing a deep blue complex copper (II) ion. In this complex state, it is very easy to reduce the Cu 2+ ion to Cu+ that is produced in the form of red insoluble copper (I) oxide (Cu2O). CH3CHO + 2 Cu2+ + 4 OH CH3COOH + Cu2O + 2 H2O Warming a mixture of a few cm3 of Fehling’s solution or Benedict’s solution, to which has been added a few drops of an aldehyde, will result in the formation of a red or a green precipitate of copper (I) oxide. Ketones fail to react. RCHO CH3CHO(l) + + [O] [O] RCOOH CH3COOH(l) Distinguishing between Primary, Secondary and Tertiary alcohols Separate samples are warmed with a few drops of acidified potassium dichromate (VI). The primary and secondary alcohols react showing a colour change from orange to green. The tertiary alcohol remains unoxidised and there is no colour change. Therefore the tertiary alcohol can now be identified. The primary alcohol is oxidised to an ALDEHYDE, whilst the secondary alcohol is oxidised to a KETONE. The products of these two reactions should now be separated from their mixtures by distillation. A few drops of tollen’s reagent or benedict’s solution added to the distillates and warmed should distinguish 2 between the primary and the secondary alcohols. The distillate from the primary alcohol will show a positive result (either a silver mirror or a red ppt) when warmed with tollen’s or benedict’s showing that an ALDEHYDE had been formed as the alcohol oxidation product, whereas the secondary alcohol distillate will show no result, showing that a KETONE was formed as the alcohol oxidation product. The Nature of the C=O bond The double bond between the carbon and the oxygen atoms consists of a sigma bond and a bond. The difference between the bond in the alkene and in carbonyl compounds like aldehydes and ketones is that the differences in electronegativities between the carbon and oxygen atoms makes the carbonyl double bond polar. The oxygen atom attracts electrons in the bond towards itself as shown in the diagram below. Addition reactions across the double bond are a feature of the chemistry of aldehydes and ketones, but, unlike the case with alkenes where the addition is characterised by electrophilic addition, carbonyl compounds react by nucleophilic attack. 1. Reduction of Carbonyl Compounds a) Hydrogenation This is exactly the same reaction as hydrogenation of alkenes. Both the carbon – carbon and the carbon – oxygen double bonds are saturated in the same way by this process. The aldehyde or ketone is vaporised and mixed with the correct amount of hydrogen and passed over a nickel catalyst at 200o C. (or platinum at R.T.) CH3 CO CH2 CH3 + H2 Butanone CH3 CH(OH) CH2 CH3 butan – 2 – ol ( a SECONDARY alcohol) CH3 CH2 CH2 CHO + H2 Butanal CH3 CH2 CH2 CH2 OH butan – 1 – ol (a PRIMARY alcohol) CH2 = CH CH2 CHO + 2 H2 CH3 CH2 CH2 CH2 OH But – 3 – enal ALDEHYDES are reduced to PRIMARY alcohols whereas KETONES are reduced to SECONDARY alcohols. b) by reaction with sodium tetrahydridoborate (III) in methanol 3 Nucleophilic Addition NaBH4 is a reagent that adds hydrogen across the double bond of a carbonyl group by nucleophilic attack on the carbonyl carbon atom. The mixture is then acidified and the intermediate formed is then converted to the alcohol. This is the reverse of the oxidation reaction where the alcohol is oxidised by acidified potassium dichromate to the aldehyde or the ketone. Since the electron rich cloud around the double bond of an alkene would repel nucleophiles, this reagent is specific to the double bond of the carbonyl group and leaves the alkene double bond untouched. The mechanism involves the initial attack on the carbonyl carbon by the nucleophile, H, from the NaBH4. Sodium tetrahydrido borate (III) is in aqueous or alcoholic solution. The water provides the proton to attach onto the carbonyl oxygen. Overall, the reaction is NUCLEOPHILIC ADDITION. Aldehydes form primary alcohols, whilst ketones form secondary alcohols. A similar result can be obtained if the aldehyde or ketone is first vaporised and then mixed with the correct volume of hydrogen and passed over a heated nickel catalyst at 200 0C. NaBH4 will NOT attack the carboncarbon double bond in an alkene because this bond is not polar. 4 2. Another Nucleophilic Addition to Carbonyl compounds The carbon – oxygen double bond is unsaturated and so is able to undergo addition reactions. The electron cloud is unevenly distributed over the bond and is more concentrated over the oxygen atom because of its electronegativity whereas it is evenly distributed over the carbon – carbon double bond in alkenes. A feature of the reactivity of the alkene double bond is, therefore, electrophilic addition. The nature of the carbon – oxygen double bond means that the initial attack on the carbonyl carbon atom is nucleophilic attack on the + carbon atom. HCN, which is usually generated by adding dilute H2SO4 to KCN at R.T., will produce an hydroxynitrile. 2 – hydroxypropanenitrile 2 – hydroxypropanenitrile has a chiral or asymmetric carbon atom and therefore has optically active isomers. In fact, all aldehydes (except methanal) and all ketones produce hydroxynitriles that have optical activity. The product formed in these reactions is not optically active, but is a racemate or a racemic mixture because attack by the cyanide ion occurs on either side of the carbonyl carbon with equal probability (since the carbonyl group is planar). From above From below 5 The hydroxynitrile compounds can then be converted into hydroxycarboxylic acids by refluxing them with concentrated hydrochloric acid. (see haloalkanes) H+ CH3 CH(OH)CN CH3 CH(OH)COOH 2 – hydroxypropanoic acid or lactic acid Lactic acid found as an energy store in muscle tissue is the d – enantiomer and is optically active. Lactic acid made in the laboratory from ethanal is optically inactive. Identifying a Carbonyl compound – a Nucleophilic Addition – Elimination Reaction • reacts with carbonyl compounds (aldehydes and ketones) • used as a simple test for aldehydes and ketones • makes orange crystalline derivatives - 2,4dinitrophenylhydrazones • derivatives have sharp, well-defined melting points • also used to characterise (identify) carbonyl compounds. A carbonyl compound will form yellow or orange crystals with BRADY’S 2,4-dinitrophenylhydrazine and is, itself, an orange solution in ethanol. REAGENT which is The first initial reaction is an addition reaction of the 2,4-dinitrophenylhydrazine to the carbonyl group. This is then followed by an elimination reaction where a molecule of water splits off from the adjacent ‘C’ and ‘N’ atoms leaving a C=N double bond. The product is a dinitrophenylhydrazone. NO2 H CH3−C NO2 H addition :NH2 NH2 NO2 CH3−C NH NH2 O OH elimination − H2O NO2 H CH3−C N NH2 NO2 + ethanal 2,4 – dinitrophenylhydrazone 6 H2O NO2 This reaction is used to tell if the compound is an aldehyde or a ketone, but it cannot be used to distinguish between the two. For this, a further test such as Benedict’s Test, or Tollen’s Reagent, or acidified Potassium Dichromate (VI) is required. (see p 2). The production of orange or yellow crystals with 2, 4 – dinitrophenylhydrazine shows that the compound being test is EITHER and aldehyde or a ketone. The aldehyde or ketone 2, 4 – dinitrophenylhydrazone product can then be used to actually identify the exact aldehyde or ketone it has been derived from. Every aldehyde or ketone 2, 4 – dinitrophenylhydrazone product has its own specific melting point and, because these compounds can be very easily recrystallised from ethanol and obtained in a high state of purity, their melting points can be determined very accurately and can be used to identify the original carbonyl compound with a high degree of certainty. The products can also be used to determine which isomer is obtained. For Example, the three isomeric chlorophenylmethanals below have very similar van der Waal’s forces between their molecules and therefore very similar boiling points. The 2, 4, dinitrophenylhydrazone products have quite widely differing melting points and so the three compounds can be readily recognised. 7