Ionic Bonds
advertisement

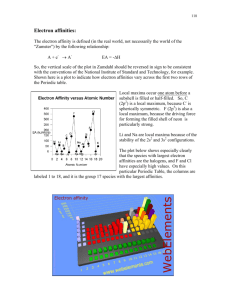
Ionic Bonds An ionic bond is a bond in which one atom gives up 1 or more electrons and another atom accepts electrons – electrons are transferred Ex) Mg and O What is the electron configuration of Mg? 2-8-2 No, it’s not stable Is it happy this way? What can it do to become more stable and have a full outer shell? Lose 2 electrons What is its electron configuration now? 2-8 Hold that for a minute and let’s talk about Oxygen. What is the electron configuration of O? 2-6 No, it’s not stable Is it happy this way? What can it do to become more stable and have a full outer shell? Gain 2 electrons What is its electron configuration now? 2-8 Okay, so Mg wants to lose 2 electrons and O wants to gain 2. Isn’t that convenient? Those 2 electrons that Mg loses have to go somewhere; they can’t just disappear, and Oxygen is happy to take them. They can form an ionic bond. Let’s draw this: Mg + O Mg+2 O-2 There are names for the positive and negative ions. The positive ion is called the cation. You can remember this because the T in cation is like a plus sign. ca+tion Which elements tend to be cations, metals or nonmetals? Metals The negative ion is called the anion. You can remember this because of the N for “negative” aNion Which elements tend to be anions? Nonmetals In MgO, which is the cation? Mg Which is the anion? O Ionic bonds usually form between metals and nonmetals. Can you find another example of two elements that would like to form an ionic bond? Remember, the charge on the final compound should be zero. Some atoms are more likely than others to form ionic bonds with each other. We had talked about a few properties of elements that describe this. What is ionization energy? The energy required for an atom to lose an electron. So, if an atom has high ionization energy is it going to be easy for it to give an electron to another atom? No So, some atoms are more likely than others to be electron donors. What about the tendency to gain electrons; what was that called? Electronegativity. So, some atoms are more likely than others to want to accept electrons. We can look at these values to predict whether 2 atoms will form an ionic bond or not. When compounds form ionic bonds, their anions and cations become arranged in a crystal lattice structure – a three dimensional pattern of ions that repeats itself.