Study Guide Answers Chapters 2
advertisement
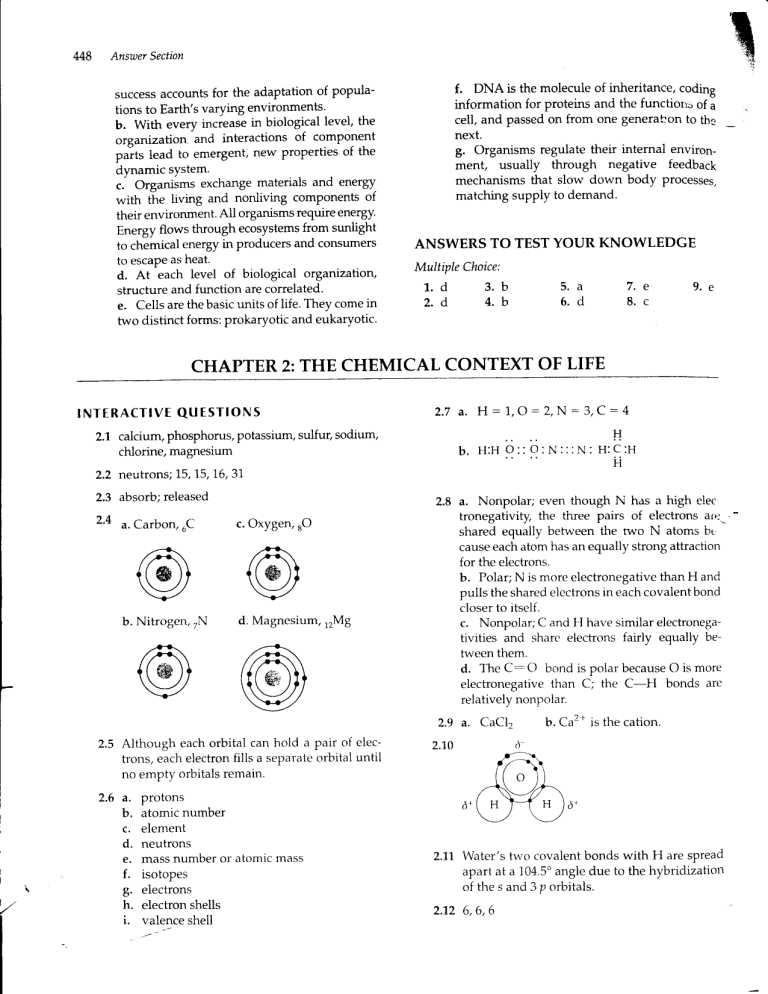
M8
AnswerSection
t, DNA is the molecule of inheritance, coding
information for proteins and the functiot.',:of q
cell, and passedon from one generalon to the
next.
g. Organisms regulate their internal environment, usually through negative feedback
mechanismsthat slow down body processes,
matching supply to demand.
success accounts for the adaptation of populations to Earth's varying environments'
b. With every increase in biological level, the
organization and interactions of component
paits lead to emergent, new ProPerties of the
dvnamic svstem.
c. Organisms exchange materials and energy
with the hving and nonliving components of
their environment. All organisms require energy.
Energy flows through ecosystems from surrlight
to chemical energy in producers and consumers
to escape as heat'
d. At each level of biological organization,
structure and function are correlated.
e. Cells are the basic units of life. They come in
two distinct forms: prokaryotic and eukaryotic.
ANSWERS TO TEST YOUR KNOWLEDGE
Multiple Choice:
L.d
2.d
5.a
6.d
3.b
4.b
7.e
8.c
9.e
CHAPTER 2: THE CHEMICAL CONTEXT OF LIFE
I N T E R A C T T V TQ U E S T I O N S
N : 3 ,C : 4
2.7a. H:7,O:2,
2.1 calcium,phosphorus,potassium,sulfur, sodium,
chlorine, magnesium
H
b . I l : H o : : o : N : : : N : H :c : H
LJ
2.2 neutrons;75,15,16,31
2.3 absorb;released
2'4 u.Carbon,
uC
b. Nitrogen,,N
c. Oxygen,rO
d. Magnesium, rzMB
2.8 a. Nonpolar; even though N has a high elec
tronegativity, the tfuee pairs of electrons ate,
shared equally between the two N atorr.s bt
cause each atom has an equally strong attraction
for the electrons.
b. Polar; N is more electronegative than H and
pulls the shared electrons in each covalent bond
closer to itself.
c. Nonpolar; C and H have similar electronegativities and sharc electrons fairly equally between them.
bond is polar becauseO is more
d. The C:O
bonds are
electronegative than C; the C-H
relatively nonpolar.
b. Ca2'''is the cation.
2.9 a. CaCl2
2.5 Although each orbital can hold a pair of elec-
d'
2.10
a'----\
trons, each electron fills a separateorbital until
n o e m p t y o r b i t a l sr e n r a i n .
'"'
a.
b.
c.
d,
e.
f.
g.
h.
i.
protons
atomic number
element
neutrons
massnumber ot'atomic mass
isotopes
electrons
electron shells
valence shell
/.,'--\\
tl
t\
t( o ll
z1\_7/-\
-f-{
6'( H
\_-/
H )a
\_--l
2.11 Water's two covalent bonds with H are spread
apart at a 104.5'angle due to the hybridization
of thes and3p orbitals.
2.126,6,6
AnswerSection
SUGGESTEDANSWERSTO STRUCTURE
YOUR KNOWLEDGE
c. The valence of an atom is most related to
the chemical behavior of an atom because it is
an indication of the number of bonds the
atom will make, or the number of electrons
the atom must share in order to reach a filled
valence shell.
3. Ionic and nonpolar covalent bonds represent
the two ends of a continuum of electron sharing
between atoms in a molecule. In ionic bonds the
electrons are completely pulled away from one
atom by the other, creating negatively and
positively charged ions (anions and cations). In
nonpolar covalent bonds the electrons are
equally shared between two atoms. Polar covalent bonds form when a more electronegative
atom pulls the shared electrons closer to it, producing a partial negative charge a5sociated
with that portion of the molecule and a partial
positive charge associated with the atom from
which the electrons are pulled.
1.
Particle
Proton
Neutron
Electron
Charge
TI
0
-l
Mass
Location
1 dalton
nucleus
l dalton
nucleus
orbitals in
electron shells
negligible
2. a. The atoms of each element have a characteristic number of protons in their nuclei, referred
to as the atomic number.In a neutral atom, the
atomic number also indicates the number of
electrons. The massnumber is an indication of
the approximate mass of an atom and is equal
to the number of protons and neutrons in the
nucleus.
The atomic massis equal to the mass number
and is measured in the atomic mass unit of daltons. Protons and neutrons both have a mass of
approximately 1 dalton.
mass number and --_
atomicweight
atomic numbe ,
ANSWERSTO TEST YOUR KNOWLEDGE
MultipleChoice:
1.b
-\
--?
6.e
7.d
8.b
9.e
10. c
.tA
rz.
J,d
"
b. The aalence,an indication of bonding caPacity, is the number of unpaired electrons that an
atom has in its valence shell.
449
4.e
5.a
11.
12.
L3.
14.
15.
b
c
c
b
e
16.
17.
18.
19.
20.
21. b
22.
23. d
24. e
25. e
c
d
b
a
c
CHAPTER 3: WATER AND THE FITNESS OF THE ENVIRONMENT
polar water molecules
absorbed
released
specificheat
heat of vaporization
evaporative cooling
q
solar heat
b'
h . rain
l.
ice forms
3.2 a.
b.
c.
d.
e.
f.
I N T E R A C T I V EQ U E S T I O N S
3.1
J.J
t:a'..
,*
.lf
a. olive oil:
hydrophobic
b. sugar:
c. salt:
d. candle wax:
hydrophilic
hydrophiiic
hydrophobic
mostly
nonPolar
polar
ionic
nonpolar
3.4 a. The molecular mass of C3H6O3is 90 d, the
combined atomic massesof its atoms. A mole of
w
AnswerSection
450
a. Bicarbonateacts as a base to acceptexcess
H* ions when the pH starts to fall; the reaction
moves to the left.
b. When the pH rises,H* ions are donated by
carbonic acid, and the reaction shifts to the
right.
lactic acid = 90 g. A 0.5 M solution would requiel2mol or 45 g.
b. 3.01x 1023
J.J
lH*l
toH-l pH
Acidic, Basic, or Neutral?
10-3
10-'
J
acidic
L0-n
10-'
8
basic
l0-
1.0-'
7
neutral
10-'
10-t'
1
acidic
3.6 carbonic acid
bicarbonate
H2CO3+HCO3-+
H+donor
3.7 a. COz + H2O .: H2CO3: HCOr- + 11+
L:rcreasing[CO2]wiil drive thesereactionsto the
right, increasing[H* J.
b. HCo3-.----'Co32-+ H+
Increasing [H*] will drive this reaction to the
left, thus decreasing[COr2 ].
c. With less CO.'- available to react with
Ca2*, calcificationrates would be expectedto
decrease,as was shown in the study by Chris
Langdon and colleagues.
hydrogen ion
H*
H+ acceptor
SUGGESTEDANSWERS TO STRUCTUREYOUR KNOWLEDGE
1.
measures
M-t'
I \
'/
ltoe'ttH'] |
if high",
if
if highei
lr|,1 [H+l=loH, 'o\
E
@
r;#,1 @
i;
may be
"i
which
u".i,,,*
ry
@
i;
regulated by
calculated by
is
dissociatesreversibly
F"i#;rfi-l
i. Floating ice insulates bodies of water so
they don't freeze solid.
j. Versatilesolvent
k. Polar water molecuies surround and dissolve ionic and polar solutes.
2. a. Cohesion, adhesion
b. A water column is pulled up through plant
vessels.
c. Heat is absorbed or releasedlt'hen hydrogen
bonds break or form. Water absorbs or releases
a large quantity of heat for each degree of temperature change.
d. High heat of vaporization
e. Solar heat is dissipated from tropical seas'
t. Evaporative cooling
g. Evaporation of water cools surfacesof plants
and animals.
h. Hydrogen bonds in ice space water molecules apart, making ice less dense.
ANSWERSTO TEST YOUR KNOWLEDGE
MultipleChoice:
1.c
2.a
3.e
4.e
s.b
6.d
'/. e
8.b
9.e
1.0.d
11. b
12. d
L3. c
14. c
15. e
'1.6.
17.
L8.
L9.
20.
a
b
d
d
e
2L. b
,',
a
AnswerSection
457
CHAPTER 4: CARBON AND THE MOLECULAR DIVERSITY OF LIFE
different bonding sequence and very different
properties. Maleic acid and fumaric acid are
geometric isomers whose double bonds fix the
spatial arrangement of the molecule. Maleic acid
is the cls isomer; both groups are on the same
side of the double bond. Although
the
enantiomersL- and o-lactic acid look similar in a
flat representation of their structures, they are
not superimposable.
INTERACTIVE QUESTIONS
4.1 A variety of organic compounds were Produced in Miller's apParatus, which attempted
to simulate conditions on early Earth. The abiotic synthesis of organic compounds may have
been a first step in the origin of life.
4.2 Ethanol and dimethyl ethea structural isomers,
have the same number and kinds of atoms but a
SUGGESTEDANSWERSTO STRUCTUREYOUR KNOWLEDGE
F.r'*l
1.
-----7have same have different
molecular
I
I
I---- shaPes I
I molecular I
I formulae I
A*\
due to
I structural| | geometric| | enantiomers
I
lito*"trllisomer:
different
arrangement
have different
of
have differcnt
different
three-dimensional
shapes
arS
spatial
arrangements
due to
placement of atoms
around
asymmetric carbon
2.
Molecular
Functional
Group
Formula
-oH
Hydroxyl
Names and Characteristics of Organic Compounds
Containing Chemical CrouP
Alcohols;poiar group
Aldehydeor ketone;polar grouP
Carbonyl
Carboxyl
Amino
-COOH
Carboxylic acid; release l{
-NHz
A m r n e s ;b a s i c ,a c c e p tH -
-SH
Sulfhydryl
-oPo.2
Phosphate
-CH.
Methyl
Thiols; cross-links stabilize protein structttre
Organic phosphates; involved in energy transfers, adds negative charge
Methylated compounds; addition may alter functron
ANSWERSTO TESTYOUR KNOWLEDCE
Matching
MultipleChoice:
L.c
2.b
3.e
4.a
5.d
5.a
r''/'
/.4
8.d
9.c
L. a,c
2. b,f
3. a,d,e
4.e
6. a,c,d,e
/. o,r
8.e
9. a,c
10. a,d
11. e
'1.2.
c
1tt
452
AnsuterSection
CHAPTER 5: THE STRUCTUREAND FUNCTION OF MACROMOLECULES
5.5 a. fats, triacylglycerides
b. phospholipids
c. glycerol
d. fatty acids
e. unsaturated:has someC:C bonds
t. saturated: no C:C,
all possible C-Ft
bonds
g. phosphategroup
h. cell membranes
i. steroids
j. animal cell membrane component (cholesterol),hormones
I N T E R A C T I V EQ U E S T I O N S
5.1 a.
b.
c.
d.
e.
hydroxyl
carbonyl
aldose
ketose
rings
I
Hto
Gr-ucosr
5.5 a.
oH'
Glucosr
H
I
CH3
H-N-c-c - (frb
\--l
I ll
HO
1A
alanine
glycosidic g
linkage
o
serine
riltil
HO
dipeptide
a. monosaccharides
b. serine's R group is polar; alanine's R group
is nonpolar
c. a polypeptide backbone
b. (cH2o)"
c. energycompounds
carbon skeletons, monomers
glycosidic linkages
disaccharides
polysaccharides
glycogen
animals
starch
cellulose
chitin
J./
5.4
Warenoutslot crll
Hydrophilic
head
Hydrophobic
tails
WATER INSIDECELL
I ll
HO
?"
HO
d.
e.
f.
g.
h.
i.
j.
k.
l.
-*- l-. - o*t ------>
H CH3
H CH2
rttl
H_N_C-C-N_C-C_OH
Malrosp
J.J
H CH2
a. hydrogen bond
b. hydrophobic and van der Whals interactions
c. disulfide bridge
d. ionic bond
These interactions between R groups produce
tertiary structure.
5.8 a. A change in pH alters the availability of H*,
OH-, or other ions, thereby disrupting the
hydrogen bonding and ionic bonds that marntain protein shape.
b. A protein in an organic solvent would turn
inside out as the hydrophiiic regions became
clustered on the inside of the molecule and the
hydrophobic regions interacted with the nonpolar solvent.
c. The return to its functional shape indicates that a protein's three-dimensional structure is intrinsically determined by its primary
structure-the sequence of its amino acids.
Answer Section
I
I PRorEnrs
5.9
t-
ur" oolvmer-s o1
function
results from
hydrogen bonds within
polypeptide backbone
hydrophobic, van der Waals
interactions.H bonds, ionic
bonds, disulfide bridges
made of
several polypeptide
subunits
b. pyrimidine; base has single ring
c. DNA; 2nd carbon in sugar is lacking O
5.10 DNA-+RNA+Protein
5.11 a.
Nitrogenous
base
5
9Ht
Phosphate
SrouP
Attachment site
for phosphate of
next nucleotide
//"
oH H
Deoxyribosc
5.\2
are PolYmers of
----+
___--tsa
!d":l
ked bv
-1-^i-, )r
may bc
l-..-il
r"'^ \
,./ consistnf \
I pentose |
I
-'l '"gu'; u"",\-1PnosPlratt'
t'o*
{l-t
-l
f
--7.s-
Jitnir
phosphate-sugar
backbone
,either -
inc | | purill
pyrimidine
Putitt"
I
ldeoxyriboscl
---T
rrs
cytoslne
thymine
uracil
(in RNA)
with
guanrne
adenine
rihoseI
provr
in
6NAl
f.-lRNilf *rr_l F;;; ^ L ,L:--r
replication
| | nr'.:n l-'s
453
q
454
Answer Section
SUGGESTED ANSWERS TO STRUCTURE
YOUR KNOWLEDGE
L. The primary structureof a protein is the specific,
genetically coded sequenceof amino acids in a
polypeptide chain. The secondary structure involves the coiling (crhelix) or folding (Bpleated
sheet) of the protein, stabilized by hydrogen
bonds along the polypeptide backbone. The
tertiary structure involves interactionsbetween
the side chains (R groups) of amino acids
and producesa characteristicthree-dimensional
shape for a protein. Quaternary structure occurs in proteins composed of more than one
polypeptidechain.
,
a.
b.
c.
d.
e.
f.
g.
amino acid (glycine)
fatty acid
nitrogenous base,purine (adenine)
glycerol
phosphate group
sugar(pentose,ribose)
sugar (triose)
L. b,d
2.a
3. c,e,f
4.f,9
5.C
6.a
7. e, f
8.b
ANSWERS TO TEST YOUR KNOWLEDGE
Matching:
1.A
2,8
3.D
4.C
5. C
5.D
7.8
8.C
9.A
10. A
11. c
12. d
13. b
'1.4.
c
1s. d
15. a
17. c
18. b
19. c
20. b
Multiple Choice:
1.e
2.c
3.a
4.e
5.c
6.b
/. c
8.a
9.d
1.0. c
2t. d
22. a
23. d
24. e
CHAPTER 6: A TOUR OF THE CELL
INTERACTIVI QUESTIONS
6.1. a. the study of cell structure
b. the intemal uitrastructure of cells
c. the three-dimensional surface topography
of a specimen
d. Light microscopy enables the study of living cells and may introduce fewer artifacts than
do TEM and SEM.
6.2 a. a phospholipid bilayer with the hydrophobic
tails clustered in the interior and the phosphate
heads facing the hydrophilic outside and inside
of the cell; proteins are embedded in and attached to the membrane
b. 702,or 100 times the surface area
c. 103,or 1,000times the volume
6.3 The genetic instructions for specific proteins are
transcribed from DNA into messenger RNA
(mRNA), which then passes into the cytoplasm
to complex with ribosomes where it is transIated into the primary structure of proteins.
6.4 a. smooth ER-in different cells may houst:
enzymes that synthesize lipids; meiabolize
carbohydrates; detoxify drugs and alcohol;
store and release calcium ions in muscle cells
b. nuclear envelope-double membrane that
enclosesnucleus; pores regulate passage of materials
c. rough ER-attached ribosomes produce
proteins that enter cisternae; produces secretory proteins and membranes
d. transport vesicle-carries products of EII
and Golgi apparatus to various locations
e. Colgi apparatus-processes products of ER;
makes polysaccharides,packap;esproducts in
vesicles targeted to specific locations
f. plasma membrane-selective barrier that
regulates passage of materials into and out of
the cell
g. lysosome-houses hydrolytic enzymes to
digest macromolecules
458
AnswerSection
CHAPTER 8: AN INTRODUCTION TO METABOLISM
e. The negatively charged phosphate groups
are crowded together, and their mutual repulsion makes this area instable. The chemical
change to a more stable state of lower free energy accountsfor the relatively high release of
energy.
T N T E R A C T TQ
VE
UESTIONS
capacity to cause change
kinetic
motion
potential
position
conserved
created nor destroyed
first
transformed or transferred
i - entropy
k. second
8.1 a.
b.
c.
d.
e.
f..
gh.
i.
8.5 a. free energy
b. transitionstate
c. EA (free energy of activation) without
enzyme
d. Ee with enzyme
e. AG of reaction
8.5
8.2
Systemwith
Systemwith
High FreeEnergy Low FreeEnergy
Stability
low
high
Spontaneous
change will be
change will not be
Equilibrium
moves toward
rsat
Work capacity high
/&
\ -:vr
Substra
-*:$
low
8.3
FREEENERGY
measure of
c
determined bv
Enzyme'substrate
complex
Substrates
converted to
products
Enzyme
energy available
to do work
A-FIis
f is
AS is
&
Produc
8.7 A competitive inhibitor would mimic the shape
of the substrates and compete with them for the
active site. Anoncompetitive inhibitor would be
a shape that could bind to another site on the enzyme molecule and would change the shape of
the active site such that the substrates could no
longer fit.
8.4 a. adenine
b. ribose
c. threephosphategroups
d. A hydrolysis reaction breaks the terminal
phosphatebond and releasesa molecule of inorganicphosphate:ATP+ H2O + ADP + @i
8 . 8 ATP would act as an inhibitor to catabolic
pathways, slowing the breakdown of fuel molecules if sufficient energy is available in the cell.
ATP may act as an activator of anabolic pathways that store resources in more complex
molecules.
459
AnswerSection
given time. Metabolic control also occurs
tfuough allostericregulation and feedbackinhibition. The compartmentalorganization of a cell
facilitatesa cell'smetabolism.
ANSWERSTO STRUCTURE
SUGGESTED
YOURKNOWLEDGE
1. Metabolism is the totality of chemical reactions
that take place in living brganisms' To create
and maintain the structural order required for
life requiresan input of free energy-from sunlight ior photosynthetic organisms and from
eiergy-ti& food moleculesfor other organisms'
n .eti couples catabolic, exergonic reactions
(-AG) wiih anabolic, endergonic reactions
energyshuttle
i+lG), usingATP asthe primary
betweenthe two.
2. Enzymes are essentialfor metabolism because
thef lower the activation energy of the specific
,"u.tiot t they catalyzeand allow thosereactions
to occur extremely rapidly at a temperature
conducive to life' By regulating the enzymesit
produces,a cell caniegulate which of the myriad
of possiblechemicalreactions take place at any
ANSWERS TO TEST YOUR KNOWLEDGE
MultipleChoice:
5.c
7.a
8.e
9.e
1"0. c
L. c
j
b
3. a
4. e
J.
c
16. d
17. b
18. d
19. c
20. b
11. b
12. c
13. b
14. b
L5. e
21'. e
22. a
FiII in the Blanks:
1.
2.
3.
4.
5.
metabolism
anabolic
kinetic
allosteric
entropy
6.
7'
8.
9.
10.
free energy of activation
competitive inhibitors
coenzYmes
feedback inhibition
phosphorylated compound
CHAPTER 9: CELLULAR RESPIRATION:
HARVESTING CHEMICAL ENERGY
INTERACTIVE QUTSTIONS
9.1 C6H12Oo;6 COz; energy (ATP + heat)
9.2 a.
b.
c.
d.
e.
oxidized
reduced
donates (loses)
oxidizing agent
acccPts(gains)
9.3 a. oxygen
b. glucose
c. Some ts stored in ATP and some is relcased
as heat.
9.4 a. electron carrier (or acceptor) or oxidizing
agent
b. NADH
9.5 a. glycolysis: glucose + pyruvate'(not technicall! consideredpart of cellular respiration)
b ' c i t r i ca c i d c Y c l e
c. oxidative phosphorylation: electron transPort and chemiosmosis
i. substrate-level phosphorylation
e. substrate-levelphosphorylation
f' oxidativePhosPhorYlation
NADH
The top two arrows show electrons carried by
the
electron
to
(and ilnH2, another electlon carrier)
transport,!::t
9 . 6 a. 2 NIP
b. 2 three-carbon sugars (glyceraldehYde3-phosphate)
c. 2 NAD*
d. 2NADH +2H+
e. 4ATP
f. 2 pyruvate
pyruvate
b. Coz
c. NADH + Hd. coenzymeA
e. acetylCoA
f. oxaioacetate
g. cltrate
9.7 a.
9.8 a.
b.
c.
d.
e.
h.
i.
i'
K.
I.
m.
n.
NADH + Hco2
Cot
NADH + HATP
FADII2
NADH + H'
intermembrane sPace
inner mitochondrial membrane
m i t o c h o n d r i a lm a t r i x
electron transPort chain
NADH + H*
T, NAD*
g. FADH2
h. chcmiosmosis
i. 2H* + 7/2Oz
i' Hto
k. ATP sYnthase
l. ADP + €)i
m. ATP








