Fever following Liver Kidney Pancreas Transplantation
advertisement

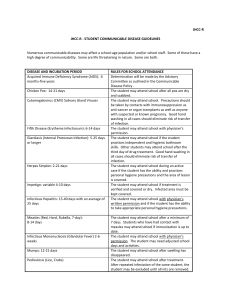
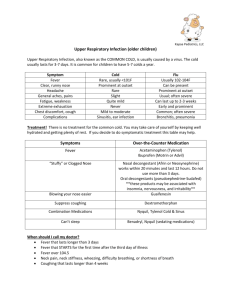
Beth Israel Deaconess Medical Center Transplant Manual Title: Fever following Liver/Kidney/Pancreas Transplantation Purpose: To provide instructions for the treatment of fevers in patients < 90 days post organ transplantation Policy Statement: Recommendations for evaluation and treatment for patients with fever posttransplantation. Definition: Fever: oral temperature >100.5 Procedure: Immediately post-op: 1) Evaluation for source of fever to include: microbiologic infection of surgical site or anastamotic injury, lungs, bladder, blood stream and line sites, stool for c. difficile toxin, as well as careful assessment for non-infectious causes of fever including medications, atelectasis, and, if indicated clinically, pulmonary embolism or other potential reasons. 2) Intravenous antibiotics will be administered based upon exposure risk, antibiogram of the ICU or Farr 10 if appropriate, at time of initial evaluation. 3) Inpatient Infectious Disease consultation will be obtained as appropriate for additional diagnostic and management support and suggestions for alternative agents in persons with documented allergy to an antibiotic or who demonstrate an allergic response to an antibiotic. 4) Once stabilized, depending upon the source of infection and organism, the patient may be followed as an outpatient by the Transplantation Infectious Disease physician. Outpatient presentation: 1) Evaluation for source of fever in patients who are discharged and return for outpatient followup should include assessment of the wound and anastamotic sites radiographically, lungs, urine, and other end-organ possibilities through a thorough history and physical examination. 2) In most cases, patients will be admitted to the hospital for more rapid diagnostic and therapeutic interventions 3) Microbiologic assessment to include viruses, including but not limited to: respiratory viruses (seasonally directed): RSV, adenovirus, parainfluenza 1, 2, and 3, and influenza A and B; reactivated or primary CMV based upon risk group (D+/R->D+/R+ or D-/R+> D-R-); EBV reactivation and B cell infection (PTLD); if appropriate, VZV, HSV or other viruses; bacterial with blood cultures and if needed, urine, stool or sputum cultures; assessment for PCP/PJP if indicated by review of systems; reassessment for reactivation of a latent infection that could not be identified pre- 1 4) 5) 6) transplantation, including reactivated HBV, latent TB infection, infectious granulomatous diseases such as histoplasmosis, blastomyces, coccidiomycoses, or other endemic fungi associated with foreign travel; reactivation of parasitic infections such as Strongyloides; and additional thorough assessment for travel or social exposures; Radiographic studies will be performed as indicated by physical and clinical findings and history/review of systems. Empiric antimicrobial coverage will be dictated by the presenting signs, symptoms and objective findings. These may include, but not be limited to: a) vancomycin for non-resistant gram positive infections; b) daptomycin or linezolid for suspected resistant gram positive infections; c) an antipseudomonal antimicrobial such as piperacillin/tazobactam or cefepime or meropenem as indicated; d) for suspicion of c. difficile colitis, metronidazole will be the first line agent; in some cases, oral vancomycin will be issued; e) for antifungal therapy, this will be determined based upon IS used, and through consultation with Infectious Disease; f) for antiviral therapy, this will be determined based upon condition and through consultation with Infectious Disease; g) for antiparasitic therapy, this will be determined based upon condition and through consultation with Infectious Disease. Antimicrobial agents and duration of the agents will be determined by clinical data and identification of the etiologic agent. The Inpatient Infectious Diseases consultation services will be consulted as needed. Vice President Sponsor: Approved by: x Liver Selection Committee Requestor Name: Original Date Approved: Next Review Date: Revised: Dianne Anderson, Sr. VP PCS Douglas W. Hanto, MD, PhD and Michael Curry, MD Co-Chairs Michael Curry, MD 12/06 1/08 Eliminated: 2