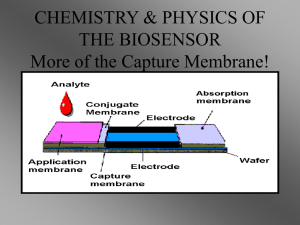
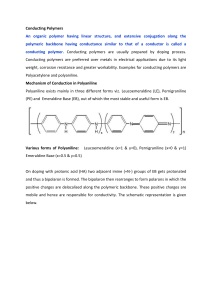
Determination of surface thermodynamic properties of
advertisement

Determination of surface thermodynamic properties of polyaniline (in conducting or non-conducting forms) by using IGC technique at infinite dilution Tayssir HAMIEH*a,b , Joumana TOUFAILYa,c a Laboratoire de Chimie Analytique, Matériaux, Surfaces et Interfaces (CHAMSI) Faculté des Sciences, Section 1, Université Libanaise, Hadeth, Beyrouth (Liban) b Institut de Chimie des Surfaces et Interfaces (ICSI-CNRS), UPR 9096 15, Rue Jean Starcky, B.P. 2488 - 68057, Mulhouse Cedex (France) c Laboratoire LACCO – ESIP, 86000, Poitiers, France Summary Many studies were devoted in our Laboratory to the determination of physico-chemical and thermodynamic properties of polymers and/or oxides by using the inverse gas chromatography (IGC) at infinite dilution. More particularly, we studied the interactions of solid substrates with some model organic molecules and their acid-base properties, in Lewis terms, by determining the acidic and basic constants. We were also interested in previous papers in studying the physico-chemical and electrical properties of conducting polyaniline (PANI). We proposed in this paper to study the surface thermodynamic characteristics of both doped polyaniline (PANI-HEBSA) and the non-conducting form (PANI-EB) using IGC technique that allowed to obtain the net retention volume Vn and then the dispersive free enthalpy Gad of n-alkanes adsorbed on PANI and the specific free enthalpy GaSP of adsorption corresponding to the specific interactions of polar molecules. By plotting GaSP of the polar molecules as a function of the temperature, we can deduce the specific enthalpy H aSP and specific entropy S aSP . Knowing H aSP of the various polar molecules, we were able to determine the acidic constant KA and basic constant KD, the two constants characterising the solid substrate and a third parameter K reflecting the amphoteric character of the oxide or polymer according to our new model: H K DN K D AN K .AN .DN Where DN and AN are respectively the donor and acceptor numbers of electrons of the solid. SP a A This expression allowed us to describe more precisely some solids (oxides and polymers) and especially the acid-base properties and specific enthalpy and entropy of adsorption of polyaniline. * Corresponding author, E. mail : tayssir.hamieh@ul.edu.lb or t.hamieh@uha.fr