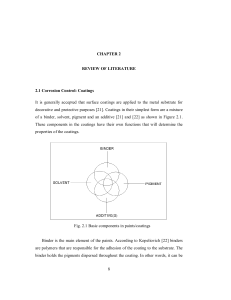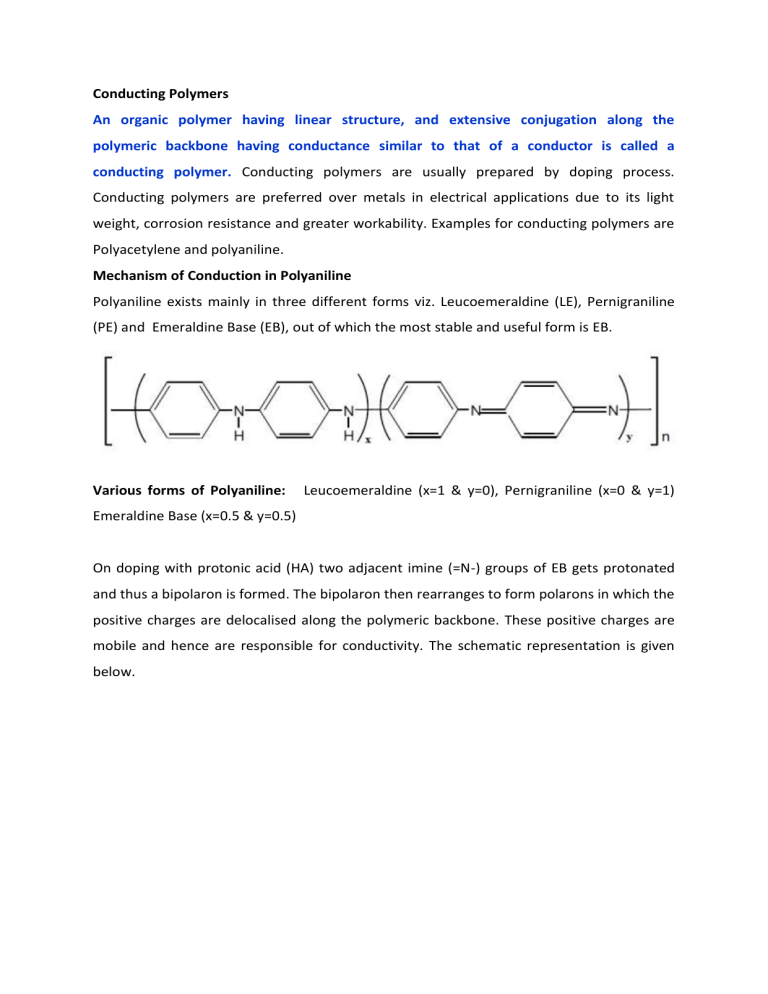
Conducting Polymers An organic polymer having linear structure, and extensive conjugation along the polymeric backbone having conductance similar to that of a conductor is called a conducting polymer. Conducting polymers are usually prepared by doping process. Conducting polymers are preferred over metals in electrical applications due to its light weight, corrosion resistance and greater workability. Examples for conducting polymers are Polyacetylene and polyaniline. Mechanism of Conduction in Polyaniline Polyaniline exists mainly in three different forms viz. Leucoemeraldine (LE), Pernigraniline (PE) and Emeraldine Base (EB), out of which the most stable and useful form is EB. Various forms of Polyaniline: Leucoemeraldine (x=1 & y=0), Pernigraniline (x=0 & y=1) Emeraldine Base (x=0.5 & y=0.5) On doping with protonic acid (HA) two adjacent imine (=N-) groups of EB gets protonated and thus a bipolaron is formed. The bipolaron then rearranges to form polarons in which the positive charges are delocalised along the polymeric backbone. These positive charges are mobile and hence are responsible for conductivity. The schematic representation is given below. Applications of Conducting Polyaniline 1. Used as bio-sensors, humidity sensors, gas sensors and radiation sensors since oxidised and reduced forms of polyaniline have different colours. 2. Used as electrode material for rechargeable batteries. 3. Used as conductive track in PCBs. 4. Used in anodic passivation of metals to control corrosion.