Enzyme Catalysis Procedure
advertisement

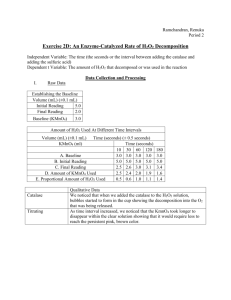
Enzyme Catalysis Procedure Day 1 - Block Day (Part A,B,C setup, D) Day 2 – Regular Day (Part C) Label one syringe with an “H” (H2O2), one with an “S” (H2SO4), one with “T” (transfer), and one syringe with a “K” (KMnO4). You will attach tubing to the “K” syringe only. Only use each syringe for the specific reagents. No mixing of syringes! Part A (Day 1) – Record Observations in Table 1 Testing Enzyme Activity 1. Add 10mL of 1.5% H2O2 into a cup and add 1 mL of catalase solution. Swirl to mix. The bubbles coming from the reaction mixture are O2, which results from the breakdown of H2O2 by catalase. Be sure to keep the catalase solution on ice at all times. 2. Discard solution in sink and rinse out and dry cup. 3. Record observations in Table 1. Effect of Extreme Temperature on Enzyme Activity 1. Add 5mL of catalase solution to a test tube and place it in a boiling water bath for 5 minutes. After 5 minutes allow the catalase to cool. 2. Add 10mL of H2O2 to a cup. 3. Using a pipette, add 1 mL of the cooled boiled catalase to the cup. Swirl to mix. 4. Discard solution in sink and rinse out and dry cup. 5. Record observations in Table 1 Part B (Day 1) – Record Data in Table 2 Establishing a Baseline – Determine the Amount of H2O2 in a 1.5% Solution 1. Add 10mL H2O2 to a cup. 2. Add 1 mL of DI water (instead of enzyme) using a pipette. 3. Add 10mL H2SO4 using the “S” syringe and mix contents by gently swirling. 4. Using the “T” syringe, transfer 5mL of the mixture into a new cup. 5. Fill up the “K” syringe with KMnO4 to 10mL. Be sure that you have added the aquarium tubing to the “K” syringe. Note the initial reading in Table 2 in the analysis section of the lab. 6. Place the cup containing the 5mL sample over a white piece of paper. Slowly add 1 drop of KMnO4 and swirl the solution mix. Continue adding KMnO4 1 drop at a time and swirl after each addition, until the solution permanently turns pink or brown. The amount of KMnO4 added is proportional to the amount of H2O2 that was present in the solution. (If you use all of the KMnO4 in the syringe, refill to the 10mL mark and continue your titration) 7. Record the final volume in the titration syringe in Table 2. 8. Discard solution in sink and rinse out and dry cups. Part C (Setup Day 1, Complete Day 2) – Record Data in Table 3 The Uncatalyzed Rate of H2O2 Decomposition 1. Dispense 25mL of H2O2 into a cup and let the cup sit uncovered for 24 hours at room temperature. 2. After 24 hours, dispense 10mL of H2O2 into a cup. 3. Repeat steps 2-6 from Part B to determine the proportional amount of H2O2 remaining. (For each calculation assume that 1 mL of KMnO4 used in the titration represents the presence of 1mL of H2O2 in the solution.) 4. Record the final volume in the titration syringe in Table 3. Part D (Day 1) An Enzyme-Catalyzed Rate of H2O2 Decomposition (10, 30, 60, 90, 120, 180, 360 seconds) 1. Add 10mL of H2O2 to a cup using the “H” syringe. 2. Add 1 mL catalase and swirl for 10 seconds. 3. Add 10 mL of H2SO4 using the “S” syringe to stop the reaction. 4. Using the “T” syringe, transfer 5mL of mixture into a new cup and label 10 seconds. Set the original sample aside – do not pour it out! 5. Repeat steps 1-4 described above for each time interval (30, 60, 90, 120, 180, 360 seconds) 6. Place the cup containing the 5mL sample over a white piece of paper. Slowly add 1 drop of KMnO4 and swirl the solution mix. Continue adding KMnO4 1 drop at a time and swirl after each addition, until the solution permanently turns pink or brown. Should the end point be overshot, remove another 5mL sample and repeat the titration. (Do not discard any of your solutions until the entire lab is completed.) 7. Record the final volume in the titration syringe in Table 4. 8. Be sure to refill your “K” syringe to 10mL before each titration. 9. Graph your results in the Data section for the lab. Plot the amount of H2O2 used on the Y-axis and the time, in seconds, on the X-axis. Be sure to properly label your graph.