Enzyme Catalysis _Lab Write
advertisement

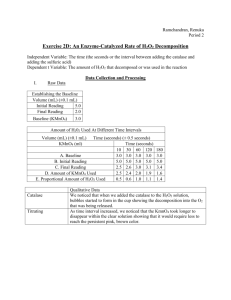
LAB 2: Enzyme Catalysis I. TITLE AND OBJECTIVES: Discuss the four parts of the lab: a. Observing Catalysis Behavior (catalase, heating, & liver). b. Establishing a Base Line c. Determining Rate of Reaction without an enzyme d. Determining Rate of an Enyzmatic Reaction when the Substrate is exposed to Catalase at Different intervals. e. State your prediction for Part C & D f. Briefly State your Results II. MATERIALS AND METHODS: In four paragraphs summarize the procedure for each of the four parts of the lab. a. Observing Catalysis Behavior (catalase, heating, & liver). b. Establishing a Base Line c. Determining Rate of Reaction without an enzyme d. Determining Rate of an Enyzmatic Reaction when the Substrate is exposed to Catalase at Different intervals. III. ***RESULTS/DATA/OBSERVATIONS: This should be divided into four clear sections with Headings A. Title of this Section: TEST OF CATALASE ACTIVITY One sentence summary of this part of the lab. Followed by data table below. TABLE 1: Test of Catalase Activity Under Various Conditions Test H2O2 WITH CATALASE Observations H2O2 WITH BOILED CATALASE H2O2 WITH LIVER B. Title of this Section: ESTABLISHING A BASE LINE One sentence summary of this part of the lab. Followed by data table below. TABLE 2: Base Line Calculations Data Initial Reading of Burette Recording (mL) Final Reading of Burette Base Line (Final – Initial) C. Title of this section: UNCATALYZED RATE OF H2O2 DECOMPOSITION One sentence summary of this part of the lab. Followed by data table below. TABLE 3: Uncatalyzed H2O2 Decomposition Data Initial Reading of Burette Recording (mL) Final Reading of Burette Amount of KMnO4 titrant Amount of H2O2 spontaneously decomposed (Base Line from Table 2 – KMnO4) % of H2O2 which spontaneously decomposed in 24 hours (ml baseline – ml 5 or 6 days)/ ml baseline x 100 ____________% D. Title of this section: TIME COURSE DETERMINATION One sentence summary of this part of the lab. Followed by data table & Graph TABLE 4: TIME COURSE DETERMINATION OF CATALASE KMNO4 10 30 60 90 sec sec sec. sec BASE LINE (from table 2) Initial Reading 120 sec. 180 sec. Final Reading Amount of KMnO4 consumed (Final – Initial) Amount of H2O2 (Baseline – Amount of KMnO4) TABLE 5 SAMPLE DATA TIME COURSE DETERMINATION OF CATALASE 360 sec. KMNO4 10 sec 30 sec BASE LINE (from table 2) Initial Reading 3.4 3.4 60 sec. 3.4 120 sec. 3.4 180 sec. 3.4 360 sec. 3.4 8.6 10.2 11.2 11.5 11.6 11.6 Final Reading 6.1 8.6 10.2 11.2 11.5 11.6 Amount of KMnO4 consumed (Final – Initial) Amount of H2O2 (Baseline – Amount of KMnO4) 2.5 1.6 1.0 .3 .1 1 drop .9 1.8 2.4 3.1 3.3 3.4 One sentence Summary of Graph Graph of the Data: X Axis: Time in seconds Y Axis: Amount of H2O2 used Title of Graph: Amount of H2O2 Decomposed by Catalase for Different Time Intervals IV. ***ANSWERS TO QUESTIONS: In this section, put answers to the questions in your lab book. Write Question & Answer out 1. From this formula Change y/Change X determine the initial rate of the reaction and the rates between each of the time points. Record the rates in the table below. Rate of Reaction Initial 10 to 30 30 to 60 60 to 90 90 to 120 120 to 180 to 0 to 10 180 360 RATES 2. When is the rate the highest? Explain why. 3. When is the rate the lowest? For what reason is the rate low? 4. Explain the inhibiting effect of sulfuric acid on the function of catalase. Relate this to enzyme structure and chemistry. 5. Predict the effect that lowering the temperature would have on the rate of enzymatic activity. Explain your prediction. 6. Design a Controlled Experiment to Test the Effect of Varying pH, temperature, or enzyme concentration. V. ***DISCUSSION: First Paragraph: Restate objective of each part of lab and summarize results for each section. Second Paragraph; Discuss Data from Table 1. Use words used in your data table. Was your hypothesis correct? Yes or no and why? Third Paragraph: Discuss Data from Table 3. How much H2O2 spontaneously broke down. Did this meet your prediction? Why or why not? Fourth Paragraph: Discuss Data from Table 4 & your graph & Rate of Reaction. Did the data make sense? Explain your numbers. If there were errors can you explain what happened? What direction was the graph? Did this make sense? Was your object met? Fifth Paragraph: Discuss Errors in lab. What went wrong & how can it be improved? Sixth Paragraph: Extend this lab. What implications does studying enzymes have on your life? Can you think of another experiment that could be done to benefit society.