1.2a CHEMISTRY OF LIFE
advertisement

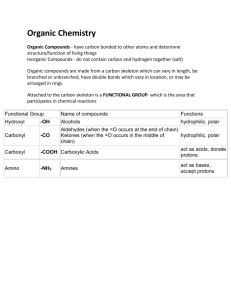
CHEMISTRY OF LIFE Flowers emit a chemical perfume that attracts butterflies, but the plant also makes a noxious chemical in its leaves which discourages the butterfly from laying her eggs there. Insects interact via chemical messages that range from “stay away” to “come mate with me”. The rattlebox moth can secrete a noxious chemical, one that is particularly distasteful to the spiders that prey on this insect. This moth is a native of Central Florida. Its name comes from the rattlebox plant, the source of the moth’s defensive chemical. This chemical also has an important role in its mating strategy. While the moth was a caterpillar, it ate the leaves from the rattlebox plant and stored this chemical in its body. While both male and female caterpillars contain this chemical, the female moth receives an extra dose at mating. During the eight hour copulation, the male passes a large mass of sperm, nutrients, and this chemical to the female, supplying additional protection for her and for their offspring. Only a human bridegroom would buy life insurance for his bride. This classy moth gives a gift she can really use-- a life insurance policy that pays off every time her life is in danger. During the courtship dance, the male moth release is into the air puffs of this chemical; the female, sensing it, can assess how much of this chemical he has. There are some kinds of chemical signaling in humans as well. For instance, chemicals in the armpit of a male can apparently regularize a female companion’s ovulatory cycle. Chemicals play many more roles in life than signaling. Chemicals make up our bodies as well as the bodies of other organisms, and they also make up the physical environment. To understand biology, we should first look at where it all begins: chemistry. ATOMS TO COMPOUNDS An atom with a certain number of protons (electrical charges) is called an element. Although life requires 25 chemical elements, 96% of the human body is composed of just four elements. They are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). Most of the other 4% of elements in our body are calcium, phosphorus, potassium, sulfur, sodium, chlorine, and magnesium. These elements are involved in important functions such as bone formation, nerve signaling, and DNA synthesis. Trace Elements Trace elements are those which are present in only tiny quantities. Examples include Iron, Iodine, Fluorine, Zinc, and Manganese. Some trace elements, such as iron, are needed by all forms of life. Iron makes up only 0.004% of your body mass but it is vital for transporting the oxygen in your blood. Others trace elements, such as iodine, are only required by certain species. The average human needs about 0.15 mg of iodine each day. Iodine is an essential ingredient of a hormone produced by the thyroid gland, which is located in the neck. An iodine deficiency in the diet causes the thyroid gland to grow to abnormal size, a condition called goiter. It can easily be reversed by iodine supplementation. Iodine is commonly added to table salt to prevent the formation of goiters. Adding iodine to table salt has reduced the incidence of goiter in many countries. Unfortunately, iodized salt is not available everywhere, and goiter still effects many people in developing nations. 1 CHEMISTRY OF LIFE Iodine is just one example of a trace element added to food or water to improve health. For more than 50 years, the American Dental Association has supported fluoridation of community drinking water supplies as a public health measure. Fluoride is a form of the element fluorine which is found in the earth’s crust in small amounts in all water sources. Fluoride is added to water in some communities as part of the water treatment process to raise levels to a concentration that can reduce tooth decay. Chemicals are added to food to help preserve it, make it more nutritious, or simply to make it look better. Iron, for example, is a trace element that is commonly added to foods. You can actually see the iron that has been added to a fortified cereal by crushing the cereal and then stirring a magnet through it. Vitamins are also frequently added to cereal. A vitamin consists of more than one element and is an example of a compound, which we will consider next: Elements combine to larger units called compounds. A compound is a substance containing two or more elements in a fixed ratio. Compounds are much more common than pure elements. In fact, few elements exist in a pure state in nature. Many compounds consist of only two elements; for instance table salt (sodium chloride) has equal parts of the elements sodium and chlorine. Pure sodium is a metal and pure chlorine is a poisonous gas. Chemically combined, however, they form a common seasoning. This example shows the emergence of novel properties with a higher level of structural organization. We will see this in biology to, as we study the adaptations of organisms as they evolved. Most of the compounds in living organisms contain at least three or four different elements, mainly carbon, hydrogen, oxygen, and nitrogen. Vitamin K, for example, is formed of just carbon, hydrogen, and oxygen. Proteins are compounds containing carbon, hydrogen, oxygen, nitrogen, and a small amount of sulfur. Different arrangements of the elements determine unique properties for each compound. There are two groups of compounds in our bodies; organic and inorganic. INORGANIC COMPOUNDS are not made of carbon atoms. 1. SALTS are found in body fluids. They are needed for muscle contraction and nerve conduction. 2. WATER The body is about 70% water. All of our body’s chemical reactions require it. It keeps the body from overheating It also prevents drastic changes in temperature. If you have ever burned your finger on a metal pot while waiting for the water in it to boil, you know that water heats up much more slowly than metal. Water has a better ability to resist temperature change than most other substances. Earth's giant water supply moderates temperatures, keeping them within limits that permit life. A large body of water can store a huge amount of heat from the sun during warm periods. At cooler times, heat given off from the gradually cooling water can warm the air. That's why coastal areas generally have milder climates than inland regions. Water's resistance to temperature change also stabilizes ocean temperatures, 2 CHEMISTRY OF LIFE creating a favorable environment for marine life. And at 70% of your body weight, water helps moderate your internal temperature. Liquids vaporize into a gas when some of their molecules move fast enough. When heat is applied to a liquid, it makes the molecules move faster and bump into each other, causing the hydrogen bonds break, allowing vaporization to occur. Another way water moderates temperatures is by evaporative cooling. When a substance evaporates, the surface of the liquid remaining behind cools down as the hottest molecules leave. Evaporative cooling helps prevent land-dwelling organisms from overheating. Evaporation from a plant’s leaves keeps them from becoming too warm in the sun, just as sweating helps to dissipate our excess body heat. On a much larger scale, the evaporation of surface waters cools tropical seas. Water is the solvent of life A solution is a liquid consisting of a uniform mixture of two or more substances. The dissolving agent is the solvent and the substance that is dissolved is the solute. Water is the solvent inside all cells, in blood, and in plants, and it dissolves an enormous variety of solutes necessary for life. The chemistry of life is sensitive to acidic and basic conditions In water solutions, a very small percentage of the water molecules actually break apart into ions. The ions formed are called hydrogen ions (H+) and hydroxide ions (OH-). The proper balance of these ions is very critical for the proper functioning of an organism. When there are excess hydrogen ions (H+), the solution is called an acid. One example of a strong acid is the hydrochloric acid found in your stomach. A solution that has excess hydroxide ions (OH-) is called a base. Ammonia is an example of a base. We use the pH scale to describe how acidic or basic a solution is. The scale ranges from 0 (most acidic) to 14 (most basic). Pure water and other solutions that are neither acidic nor basic are said to be neutral; they have a pH of 7. The pH of the solution inside most living cells is close to 7. Even a slight change in pH can be harmful. Acid rain threatens the environment Imagine arriving for a long awaited vacation at a mountain lake only to discover that since your last visit a few years ago, all fish and other forms of life in the lake have perished because of increased acidity of the water. Over the past quarter-century, thousands of lakes in North America, Europe, and Asia have suffered that fate. This problem is due to acid rain. Acid rain is defined as rain having a pH well below 7. Acid rain results mainly from sulfur oxides and nitrogen oxides in the air. These elements react with water vapor in the air to form sulfuric acid and nitric acid, which fall to the earth in rain or snow. Acid rain with a pH of 1.7 (almost as acetic as the digestive juices in the human stomach) has been recorded in Los Angeles. Sulfur and nitrogen in the air comes from the burning of fossil fuels such as coal, oil, and gas. Electrical power plants that burn coal produce more of these pollutants than any other single source. The effect of acid in lakes is most pronounced in the spring, as snow begins to melt. The surface snow melts first, drains down, and sends much of the acid that has accumulated over the 3 CHEMISTRY OF LIFE winter into lakes and streams all at once. This acid surge hits when fish and other forms of aquatic life are producing eggs and young, which are especially vulnerable to acetic conditions. Acid rain has also taken a toll on forests. When acid precipitation falls on land, it washes away mineral ions, such as calcium and magnesium, which are essential nutrients for plant growth. At the same time, minerals such as aluminum reach toxic concentrations. In cities, acid precipitation causes a great deal of corrosion of buildings and statues. That is why laws were enacted that require reductions in emissions to help alleviate the problem. Every organism has a delicate biochemistry that needs to be maintained. One spring, a baby finch collapsed with exhaustion on my patio. Since it was exhausted, it probably wasn’t good at finding food and water yet. That means it was dehydrated and hungry. I knew to get an eyedropper and give it water with sugar in it because those are the two main things it needs right away. We discussed water, now let’s get to sugars. ORGANIC COMPOUNDS are always made of carbon, which is what our body is mostly made of. The three main types of organic compounds in our body are carbohydrates, lipids, and proteins. 1. CARBOHYDRATES are compounds that store energy for a short time (compared to fats). There are two main types of carbohydrates; simple (sugars) and complex (starches). Plants also have carbohydrates called cellulose (fiber). a) SIMPLE CARBOHYDRATES known as sugars, such as those found in candy. They are used for a quick source of energy, and they are burned off fast. The main sugar form is glucose, but there are other sugars such as sucrose, fructose, and lactose. Got milk? Most of the world's people cannot easily digest milk-based foods. Milk and other dairy products have long been recognized as highly nutritious foods, rich and proteins and minerals necessary for good teeth and strong bones. But for millions of people, those health benefits come with digestive discomfort. Such people suffer from lactose intolerance, or the inability to properly break down lactose, the main sugar found in milk. For those with lactose intolerance, the problem starts once lactose passes through the stomach and enters the small intestine. To absorb this sugar, digestive cells need to secrete an enzyme called lactase, which is necessary to break down lactose. An enzyme is a protein that breaks down larger molecules into smaller ones. Those with lactose intolerance produce insufficient amounts of the enzyme and the lactose cannot be properly digested. This leads to symptoms of nausea, cramps, diarrhea, and gas. At birth, nearly everyone produces enough lactase to digest the lactose in breast milk and dairy products. Therefore, milk provides excellent nourishment for infants. But after the age of two, lactase levels start to decline in most of the world’s populations. In the United States, 75% of African Americans and Native Americans and 90% of Asian-Americans are lactase deficient once they reach their teenage years. People of European descent are the only group that does not suffer much from lactose intolerance. The reason for this is genetic. 4 CHEMISTRY OF LIFE Lactose is widely used in everything from bottled salad dressings and lunchmeat to prescription drugs. Currently, lactose intolerance cannot be corrected by gene therapy to treat the underlying cause, but the symptoms of lactose intolerance can be controlled through diet. In many Asian cultures, beverages are made from soy or rice (which does not contain lactose) instead of milk. Other foods are prepared from milk that has been pre-treated with lactase. Lactase, in pill form, can also be taken along with food to the ease digestion. Lactose intolerance, with its interplay between genes and milk sugar, illustrates the importance of biological molecules to the functioning of living cells and to human health. In people who easily digest milk, lactose (a sugar), is broken down by lactase (a protein), which is produced by a gene made of DNA (a nucleic acid). If the gene for lactase production is not active, lactase is not present. And the presence of lactase can mean the difference between delight and discomfort when someone contemplates an ice cream sundae. The taste we describe as sweet has been a beloved sensation throughout human history. However, sugars are not the only substances perceived as sweet; there are other chemicals that can trigger the same sensation. We perceive sweetness when molecules of a substance attach to the “sweet” taste receptors on our tongue, triggering a message to the brain. Many different kinds of molecules can bind to our “sweet” taste receptors, each causing a similar message to be sent. The glucose and fructose in honey taste sweet but so does the laboratoryproduced compound called aspartamine (Equal and NutraSweet). Compared to table sugar (sucrose), fruit sugar (fructose) is four times sweeter. The chemical shape of a compound determines how well it fits into a taste receptor. Compounds that bind more tightly to “sweet” taste receptors send stronger “sweet” messages to the brain. Some artificial sweeteners are much sweeter than sucrose because their molecules fit more snugly into our sweet taste receptors than natural sugars. Neotame, a new artificial sweetener that received FDA approval in 2002, has been rated 8000 times sweeter than sucrose. Therefore, smaller quantities are needed. However, some sugar substitutes also bind to other kinds of taste receptors on the tongue. For example, a sweetener may have a bitter aftertaste because it also binds to “bitter” receptors. b) STARCH is a storage form of glucose in plants, especially potatoes and grains (wheat, corn, rice). When we eat breads, corn, rice, potatoes, and cakes, we convert it to glucose. These don’t break down to glucose as easily, so they tend to get stored and are only broken down when there is not enough glucose available. c) CELLULOSE is the most abundant organic compound on earth, and is only found in plant cell walls, and gives plant stems and leaves their firmness. Our body is unable to break down this substance, so it just passes through our digestive tract. That is what is referred to as eating fiber. It helps a person who has constipation. Foods that are high in fiber are most likely derived from plants. Fresh fruits, vegetables, and grains are rich in fiber. The fiber portion of each of these foods is the wall of each cell. The contents of each cell contain the carbohydrates which can be digested. This is one reason you should chew your food well; crushing up the cell walls will release the nutrients. 5 CHEMISTRY OF LIFE If you swallow a whole kernel of corn, it will pass right through your digestive tract without being digested. You may have heard the term cellulite referring to fat. However, there is no such thing; it is just regular fat, which we’ll talk about now. Some companies made up the term and said their cream can dissolve it: NO! 2. LIPIDS differ from carbohydrates in that they don’t dissolve in water. a) FATS AND OILS: Fats are animal lipids, and oils are plant lipids. When we ingest (eat) oils, we convert them to fats. One gram of fat stores more than twice as much energy as one gram of starch. Function of fats Fats are for long-term energy storage. They also insulate against heat loss Fat forms protective cushions around organs. 1) SATURATED FATTY ACIDS are solid at room temperature, like butter and lard. 2) UNSATURATED FATTY ACIDS are liquid at room temperature, such as vegetable oils Most plant fats are unsaturated oils, whereas most animal fats are saturated solids. Diets rich in saturated fats contribute to cardiovascular disease by promoting a condition called atherosclerosis. In this condition, the lipids deposits called plaques build up within the walls of blood vessels, reducing blood flow. b) STEROIDS are lipids that have a very different structure than fats. Steroids are formed from cholesterol, which is found in the cell membranes of our body. Examples of steroids that our body makes are estrogen and testosterone. Anabolic steroids are synthetic forms of the male hormone testosterone. Testosterone causes a general buildup in muscle and bone mass in males during puberty and maintains masculine traits throughout life. Because anabolic steroids structurally resemble testosterone, they also mimic some of its effects. The term anabolism means to “build up” substances by the body. As prescription drugs, anabolic steroids are used to treat anemia and diseases that destroy body muscle. However, some individuals abuse these drugs, with serious consequences. Overdosing may cause violent moods swings (“steroid rage”) and deep depression. The liver may be damaged, leading to cancer. It can also alter cholesterol levels and lead to high blood pressure. The use of these drugs often makes the body reduce its output of natural male sex hormones, which can cause shrunken testicles, reduced sex drive, infertility, and breast enlargement in men. Use in women causes menstrual cycle disruption and development of masculine characteristics, including facial hair. In teenagers, bones may stop growing, stunting growth. Despite risks associated with steroid use, some athletes use steroids to gain a competitive edge. Sports organizations banned their use, implement drug testing, and penalize violators. In 2003, the discovery of a new designer steroid rocked the sports world. THG is a drug modified to avoid 6 CHEMISTRY OF LIFE detection in ordinary drug testing. The drug was discovered when a track coach mailed a syringe containing a sample of it to the US Anti-Doping Agency. With that sample, the agency was able to develop a test that revealed the substance’s use among track and field athletes and professional football players, so the International Olympic Committee has begun retesting frozen and urine samples from the 2002 Winter Games. The US FDA declared THG an illegal steroid. In 2004, a British sprinter became the first athlete to be penalized for its use, with a permanent exclusion from the Olympics following his positive test for THG. Performance-Enhancing Drugs Human Biology by Sylvia Mader (page 211) Some athletes may be better at one sport than another, depending on whether their muscles contain fast or slow-twitch fibers. A natural advantage of this sort does not bar and athlete from participating in and winning a medal in a particular sport at the Olympic Games. Also, some athletes have an advantage over others due to improved equipment and special shoes, advances in sports nutrition, new weight training regimens, specialized techniques based on their particular anatomy and physiology, and almost miraculous medical treatments for injuries. Such advantages are not considered reason enough to bar an athlete from receiving a medal. However, if athletes take any number of performance enhancing drugs, they are barred from getting a medal. In the past, athletes have turned to other dangerous and potentially life-threatening techniques to improve their edge. It was fairly common for wrestlers to use severe dehydration, diuretics, and laxatives to lose weight in order to participate in a lower weight class. In blood doping, blood is removed from an athlete's body several weeks before a competition and then reinjected into the body right before the event. A heart attack or stroke can occur due to the increased blood thickness resulting from the increased amount of red blood cells. Anabolic steroids are one class of drugs commonly abused by athletes today. Although illegal, many of these drugs are available for sale on the Internet. Anabolic steroids are synthetic substances related to male sex hormones that bulk muscle mass and increased strength. When abused at high doses, they also increase hostility and aggression and cause heart disease, liver cancer, acne, eating disorders, sterility, and stunted height. Withdrawal leads to mood swings, fatigue, depression, and often suicide attempts. Despite these drawbacks, some athletes still want to use these drugs. Should they be allowed to? On the horizon are other ways that might give some athletes an advantage over their competitors. Gene therapy may soon be available to help the aging in disease repair and strengthening their skeletal muscles. The gene for insulin-like growth factor may help increase the growth of skeletal muscles in these patients. The gene for red blood cell formation can cause those with anemia to increase the number of red blood cells. Gene therapy using these genes is still in the experimental stage. When ready for use in humans, such gene therapy will be well within the guidelines for medical treatment of human disorders. But should they be used by athletes to enhance their performance? Should the international Olympic Committee outlaw the taking of any medication or the use of any procedure to enhance an athlete's performance? If so, on what basis should this decision be made? One cannot say that it is an unfair advantage because some athletes naturally have 7 CHEMISTRY OF LIFE an unfair advantage over other athletes to begin with. Should performance-enhancing drugs or procedures be outlawed on the basis of health reasons? Excessive practice alone in a purposeful decrease or increase in weight to better perform in a sport can also injure a person's health. In other words, how can you justify allowing some behaviors that enhance performance and not others? 1. Do you believe that the techniques athletes used to train and enhance their performance should be regulated in any way? Does this apply to high school athletes as well? 2. Is it acceptable for athletes to endanger their health by practicing excessively? By gaining or losing weight? By taking drugs? 3. Who should be in charge of regulating the behavior of athletes so that they do not harm themselves? We talked about carbohydrates and lipids; now let’s go on to proteins. 3. PROTEINS are compounds that make up most of our body. Our hair, nails, tissues, ligaments, cartilage, bone, tendons, muscles, and organs are made of proteins. Other proteins we have are enzymes, which function to speed up metabolic reactions and break down larger compounds into smaller ones. A protein is made from a string of amino acids. Each of our many thousands of different kinds of proteins has a unique shape that corresponds to a specific function. Other types of proteins include the anti-bodies of our immune system, hormones that coordinate bodily activities, hemoglobin in red blood cells which deliver oxygen to working muscles, transport proteins that move sugar molecules into cells for energy, storage proteins, the protein of egg white, and milk proteins which provide amino acids for baby mammals. Plants also have storage proteins for the developing embryos in their seeds. Since proteins are made of amino acids, in order to understand what a protein is, we have to talk about amino acids (AA’s). a) AMINO ACIDS are the building blocks of protein. They are tiny compounds, made of just a carbon atom and a few other atoms. Although there are many thousands of different types of proteins, they are all made up of a various combination of only 20 amino acids. They are like beads on a necklace. How they are arranged on the string determines the type of necklace. Each bead is an amino acid, and the whole necklace is the protein. A protein’s specific shape determines its function. A bunch of the same types of necklaces (proteins) woven together makes up our tissues. If a protein becomes denatured, the amino acid chain unravels, causing a loss in shape and, as a result, function. Things that can denature a protein include salt concentration, pH, and excessive heat. You can see an example of protein becoming denatured by frying an egg. Heat quickly denatures the clear protein surrounding the yoke, making them solid, white, and opaque. One of the reasons why extremely high fevers are so dangerous is that some proteins in the body become denatured and cannot function. 8 CHEMISTRY OF LIFE b) NUCLEIC ACIDS are the special types of amino acids that make up DNA and RNA. DNA makes up our genes. GENES store information about how to replicate, including how to arrange the amino acids in the new cell to form the proper proteins for the body. When a person has a genetic defect, it is because the nucleic acids are not in the exact right order. Sometimes, just one amino acid in the wrong order will cause death in a person before they are born. There are two types of nucleic acids: DNA and RNA. The genetic material that organisms inherit from their parents consists of DNA. Genes are the specific stretch of a DNA molecule that programs the amino acid sequences. An architect who spends a lot of time designing an original blueprint for a building does not take this precious document down to the dusty construction site. Instead, he makes a copy and leaves the original at home in a safe place. Likewise, DNA in the nucleus does not put its genetic information to work directly by leaving the nucleus. It works through an intermediary called RNA, which can enter the nucleus from the cytoplasm, make a copy of the gene and take it outside of the nucleus into the cytoplasm, were the protein is actually built. To do this, the information in DNA is first copied onto a strand of messenger RNA (mRNA), which is like stamping an impression in clay. Since the clay impression is not an exact copy of the original, but is instead a reverse copy, the DNA then needs to be translated before the protein is built. This is done by transcription RNA (tRNA). After the protein is built in the cytoplasm, it is either used by that cell or transported outside of the cell so it can be taken wherever else in the organism it is needed. As we stated, DNA is made up of a string of nucleic acids, which are like beads on a necklace. Now let us take off one of these nucleic acid “beads” and examine it. One nucleic acid is made up of a string of nucleotides, which is also like a miniature bead necklace. There are only four types of nucleotides (“beads”): adenine (A), thymine (T), cytosine (C), and guanine (G). Therefore, the genetic code of a very short protein may look like this: AATCAGCT. If you were to remove the last letter in that sequence, a completely different protein would form. Likewise, if you were to substitute the last letter in that sequence for a different letter, you would also get a completely different protein. And of course, if you insert additional letters, you would have a new protein. Actually, a DNA “string of beads” is actually double-stranded. Each of the nucleotides (A,T,C,G) on one strand fits like a puzzle piece into the nucleotides on the other strand. The nucleotide adenine (A) always pairs up with thymine (T), and cytosine (C) always pairs up with guanine (G)…these are called base pairs. Therefore the two strands of DNA lock together like a jigsaw puzzle. The two strands of this DNA “string of beads” are also twisted like a coiled telephone cord. This structure is called a double helix. Most DNA molecules are very long, with thousands or even millions of base pairs. One long DNA molecule may contain many genes, each one being a specific series of hundreds or thousands of nucleotides. The specific sequence of nucleotides in a gene is the information that programs the primary structure of a protein. 9 CHEMISTRY OF LIFE c) ATP is a type of protein that provides all the energy to cells. When food is broken down to glucose for energy, ATP is what is released, which is the actual energy molecule. The more ATP that is produced, the more energy we have. When we inhale oxygen, it is used in a process called respiration, which produces ATP for energy. That is why we breathe. Just remember that ATP is an energy molecule. This information will be useful in understanding proper nutrition when we get to that section. In the meantime, now that you understand what these compounds are, next we’ll talk about what a typical cell in our body looks like. 10