6th Grade Heat and Temperature Standards
advertisement
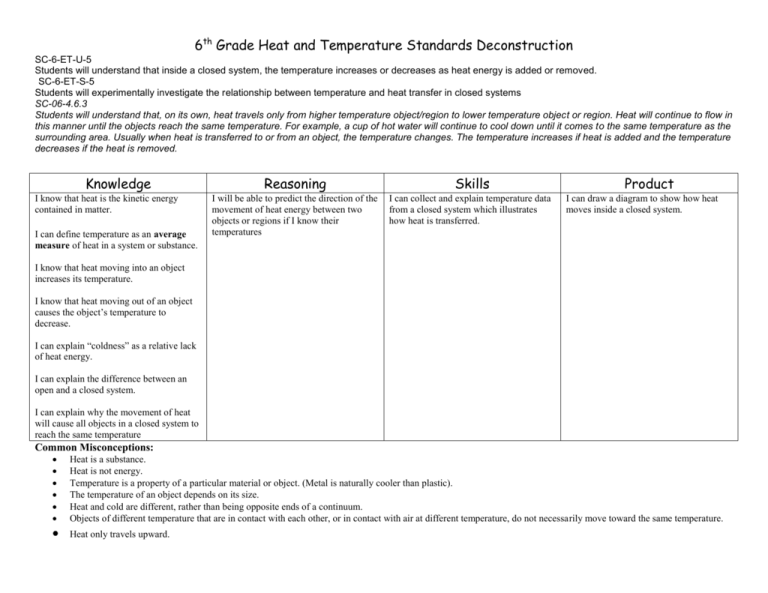
6th Grade Heat and Temperature Standards Deconstruction SC-6-ET-U-5 Students will understand that inside a closed system, the temperature increases or decreases as heat energy is added or removed. SC-6-ET-S-5 Students will experimentally investigate the relationship between temperature and heat transfer in closed systems SC-06-4.6.3 Students will understand that, on its own, heat travels only from higher temperature object/region to lower temperature object or region. Heat will continue to flow in this manner until the objects reach the same temperature. For example, a cup of hot water will continue to cool down until it comes to the same temperature as the surrounding area. Usually when heat is transferred to or from an object, the temperature changes. The temperature increases if heat is added and the temperature decreases if the heat is removed. Knowledge I know that heat is the kinetic energy contained in matter. I can define temperature as an average measure of heat in a system or substance. Reasoning Skills I will be able to predict the direction of the movement of heat energy between two objects or regions if I know their temperatures I can collect and explain temperature data from a closed system which illustrates how heat is transferred. Product I can draw a diagram to show how heat moves inside a closed system. I know that heat moving into an object increases its temperature. I know that heat moving out of an object causes the object’s temperature to decrease. I can explain “coldness” as a relative lack of heat energy. I can explain the difference between an open and a closed system. I can explain why the movement of heat will cause all objects in a closed system to reach the same temperature Common Misconceptions: Heat is a substance. Heat is not energy. Temperature is a property of a particular material or object. (Metal is naturally cooler than plastic). The temperature of an object depends on its size. Heat and cold are different, rather than being opposite ends of a continuum. Objects of different temperature that are in contact with each other, or in contact with air at different temperature, do not necessarily move toward the same temperature. Heat only travels upward.