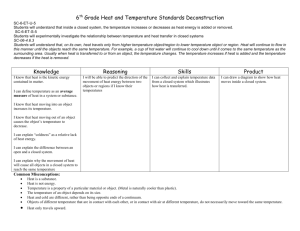
Heat Transfer 09/11/2020 Experiment: • Fill each container with the same volume of boiling water • Touch the outside of each container Predictions: • What do you think will happen? Will both containers feel the same at touch? Heat Transfer Learning Intentions • Learn what is meant with ‘heat’ and ‘temperature’ • Understand that there are several ways to ‘transfer heat’ • Investigate how heat is transferred through “conduction” Success Criteria I can: • Describe how heat moves across different materials • Successfully measure temperature over time when heat is being transferred Heat Transfer Heat is a form of energy and it is transferred from one object to another. Heat is measured in Joules (J) Temperature is a measurement of how hot something is. Temperature in measure in degrees Celsius Does blowing on hot food really make it cooler? Experiment: Heat on the Move Aim: To discover how heat moves. Heat Transfer Heat can be transferred through radiation, conduction, and convection. • Heat is always transferred from the warmer object to the cooler object Heat Energy How energy is stored? Although energy is always energy, it can be stored in different ways: • Thermal, as in a cup of tea How energy is transferred? Energy can move from one store to another and to dissipation in various different ways, such as: • Transfer from a hotter to a cooler body Conduction Conduction is the transfer of heat between two objects that are touching. When would you like to ‘loose heat’? When would you like to ‘keep’ heat? If you were to wake up in the middle of the night and go out of bed, what type of flooring do you prefer to step in? Are some materials faster than others to transfer het?