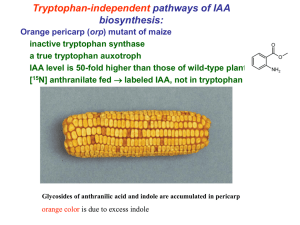
Indole-Acetic Acid Production by Root Associated Bacteria and its
advertisement

In: Auxins: Structure, Biosynthesis and Functions Editors: Andrew H. Keller and Michelle D. Fallon ISBN: 978-1-62100-504-9 ©2011 Nova Science Publishers, Inc. Chapter 7 INDOLE-ACETIC ACID PRODUCTION BY ROOT ASSOCIATED BACTERIA AND ITS ROLE IN PLANT GROWTH AND DEVELOPMENT Egamberdieva Dilfuza National University of Uzbekistan, Faculty of Biology and Soil Sciences, Tashkent, Uzbekistan Indole-3-acetic acid (IAA) is the most abundant endogenous auxin which has roles in stem elongation and root growth. The auxin level is usually higher in the rhizosphere, where high percentage of rhizosphere bacteria is likely to synthesize auxin as secondary metabolites because of the rich supplies of root exudates. The production of auxin (IAA), has been recognized as an important factor in direct plant-growth-promoting abilities of rhizosphere bacteria. They stimulate proliferation of lateral roots that increase nutrient absorbing surfaces and results in better assimilation of water and nutrients from the soil. This in turn would be expected to significantly increased the shoot and root length of plants. This review focuses on indole-3-acetic acid produced by root associated bacteria and its involvement in plant growth promotion and yield increase. Key words: indole-3-acetic acid, rhizosphere bacteria, plant growth INTRODUCTION The microbial composition in the rhizosphere is diverse and differs greatly from one plant species to another (Kloepper et al. 1989; Lugtenberg et al. 2001; Pinton et al. 2001; Marschner et al. 2001). The root associated microorganisms influence plant development, growth, and environmental adaptation, both beneficially and detrimentally (Kloepper et al. 1986; Glick et al. 1995; Lugtenberg and Kamilova 2004). Various soil microorganisms that are capable of exerting beneficial effects on plants or antagonistic effects on soil-borne pests and diseases have potential uses in agriculture to support economic crop production (Lugtenberg and Kamilova 2004; Mayak et al. 2004; Haas and Défago 2005). Some of them may be able to improve plant growth by increasing the rate of seed germination and seedling 2 Egamberdieva Dilfuza emergence, minimizing the adverse effects of external stress factors, and protecting plants from soil-borne pests and diseases (Thomashow and Weller 1996; Berg et al. 2005). It is reported that plant growth promoting rhizobacteria are able to colonize the rhizosphere, the root surface, or even superficial intercellular spaces of the plants (Kamilova et al. 2005) and will facilitate to develop a beneficial association with the plants. Increased nutrient uptake by plants inoculated with effective bacteria has been attributed to the production of plant growth regulators by the bacteria at the root interface, which stimulated root growth and facilitated greater absorption of water and nutrients from the subsoil (Haas and Défago 2005). Therefore, if the increase in the amount of nutrients taken up by the PGPB-inoculated plants were simply due to an increase in root surface area, the effect of PGPB would be entirely dependent on root development, and not on the uptake function (Lugtenberg and Kamilova 2009). Mechanisms by which bacteria are able to prevent damage caused by plant pathogens and promote plant growth include antagonism (Thomashow and Weller 1996; Bloemberg and Lugtenberg et al., 2001; 2004; Raaijmakers et al. 2009), induction of systemic resistance (Van Loon 2007), competition for nutrients and niches (Kravchenko et al. 2004; Kamilova et al. 2005), mobilization of nutrients (Lifshitz et al. 1987; Lugtenberg et al. 2001) and production of phytohormones (Costacurta and Vanderleyden 1995; Spaepen et al. 2009). This chapter presents an overview of the indole-3- acetic acid (IAA) production by root associated bacteria, provides some discussion of how bacterial IAA effect plant growth and also elucidates whether biotic and abiotic factors alter their plant growth stimulation activities. AUXINS AND ITS EFFECTS ON PLANTS Plants use a wide variety of phytohormones such as auxins, abscisic acid, cytokinins, ethylene and gibberellins, which are all relatively small molecules (Teale et al. 2006). Plant hormones play an important role in plant physiology, such as seed germination, root formation, blossom formation etc. Indole-3-acetic acid (IAA) is the most abundant naturally occurring auxin with a well-documented ability to regulate many aspects of plant development some of them include the differentiation of vascular tissues, elongation growth, apical dominance, lateral root initiation, fruit setting and ripening (Macdonald 1997; Woodward and Bartel 2005). Plants produce active IAA both by de novo synthesis and by releasing IAA from conjugates (Davies 1995; Bartel 1997). The exogenous application of auxins to alfalfa (Gruodien and Zvironaite 1971) and groundnut (Srinivasan and Gopal 1977) promoted plant growth and nodulation. Hayat et al., (2008) also observed where IAA significantly affected the length of mung bean, fresh and dry mass of roots and shoots, the number of nodules and the nitrogenase activity. It is thought that the repressive effect of salinity on germination could be related to a decline in endogenous levels of plant growth hormones or phytohormones (Zholkevich and Pustovoytova 1993; Jackson 1997; Debez et al. 2001). The salt stress reduces the supply of cytokinin from root to shoot and also the recovery of diffusible auxin from maize coleoptile tips (Itai et al. 1968). Sakhabutdinova et al. (2003) also reported that salinity resulted in a progressive decline in the level of IAA in the root system of plants. In this condition seed Indole-Acetic Acid Production by Root Associated Bacteria … 3 soaking with plant growth regulators and application of additional natural phytohormones supplied sufficient hormones for normal plant development and growth in saline conditions (Kabar 1987, Afzal et al. 2005). In other studies the exogenous application of plant growth regulators (PGRs) e.g. gibberellins (Prakash and Parthapasenan 1990; Afzal et al. 2005), auxins (Gul et al. 2000; Khan et al. 2001, 2004), cytokinins (Dhingra and Varghese 1985; Khan and Weber 1986; Gul et al. 2000) produced some benefit in alleviating the adverse effects of salt stress and they also improve germination, growth, fruit setting, fresh vegetable and seed yields and yield quality (Saimbhi 1993). In our observation the plant growth regulators such as auxin and gibberellins did reverse the growth inhibiting effect of salt-stress to a certain extent in both shoot and root (Table 1). Among plant growth regulators the IAA and gibberellins showed high stimulatory effect on the root (up to 3.1 cm) and shoot growth (up to 1.6 cm) of cucumber seedling at all tested concentrations in saline condition, compared to root (2.4 cm) and shoot (0.7 cm) length of control cucumber seedling. Gibberelin and auxin stimulated root and/or shoot growth of cucumber seedling at concentration 0.1, 0.01 µM in non saline condition as well, where zeatin had no effect on the stimulation of both shoot and root length. Other authors also reported that pre-sowing plant seeds with plant growth regulators like IAA, gibberellins alleviated the growth inhibiting effect of salt stress (Singh and Dara 1971; Datta et al. 1998; Sastry and Shekhawa 2001; Afzal et al. 2005). The use of IAA producing bacteria in crop cultivation as inoculums may significantly stimulate seed germination, root growth and yield (Mehnaz and Lazarovits 2006; Egamberdieva 2009). In our previous study (Egamberdieva 2009) the inoculation of wheat with IAA producing bacterial strains P. aureantiaca TSAU22, P. extremorientalis TSAU6, and P. extremorientalis TSAU20 significantly increased seedling root growth of wheat up to 40% and shoot growth up to 52% at 100mM NaCl compared to control plants. Table 1. The effect of different concentrations, 0, 0.1, 0.01, and 0.001 µM of gibberelin (GB), auxin (IAA) and zeatin (ZT) on the shoot and root growth of cucumber seedling in 0.6% NaCl condition (* significantly different p<0.05). Treatments Control (0.6% NaCl) IAA 1.0 0.1 0.01 0.001 GB 1.0 0.1 0.01 0.001 ZT 1.0 0.1 0.01 0.001 Root length, cm 2.4 ± 0.6 Shoot length, cm 0.7 ± 0.3 1.4 ± 0.1 1.8 ± 0.4 2.6* ± 0.9 3,1* ± 0.5 0.6 ± 0.6 0.5 ± 0.5 0.9* ± 0.5 1.2* ± 0.4 2.0 ± 0.8 2.6 ± 0.6 2.9* ± 1.0 3.0* ± 0.9 0.5 ± 0.7 0.9 ± 0.7 1.4* ± 1.5 1.6* ± 0.9 2.2 ± 1.0 2.0 ± 1.0 2.2 ± 0.7 2.5 ± 0.5 0.4 ± 0.7 0.6 ± 0.5 0.5 ± 0.6 0.6 ± 0.5 4 Egamberdieva Dilfuza In addition IAA plays also an important role in plant–pathogen interactions (Jameson 2000). Several studies reported where auxins involved in the infection process of root pathogens, such as P. ultimum (Rey et al. 2001; Le Floch et al. 2003). Gravel et al. (2007) studied the effect of IAA on the development of symptoms caused by Pythium ultimum on tomato plants and where found that low concentrations of IAA (0–0.1 mg ml-1) within the rhizosphere of plantlets increased the severity of the symptoms caused by P. ultimum, while higher concentrations (10 mg ml-1), applied either by drenching to the growing medium or by spraying on the shoot, reduced the symptoms caused by this pathogen. Fernández-Falcón et al. (2003) suggested that the exogenous application of indoleacetic acid to banana plants induce resistance to Panama disease and that the resistance induction is more effective when performed using low doses and frequent applications. Similar result obtained by Sharaf and Farrag (2004) where IAA reduced infection rate of tomato wilt caused by Fusarium oxysporum lycopersici. In their study disease suppression exerted by application of IAA, was achieved through either increasing plant growth exerting a direct harmful effect on the target pathogen and/or inducing resistance in host tissue. IAA PRODUCTION BY RHIZOSPHERE MICROORGANISMS IAA is produced through L-TRP metabolism by many of soil micro-organisms such as bacteria, fungus and algae (Sarwar and Kremer 1995). Rhizosphere microorganisms associated with various plants synthesize and release auxin as secondary metabolites because of the rich supplies of substrates exuded from the roots compared with non rhizospheric soils (Kampert et al. 1975; Lugtenberg et al. 2001; Shahab et al. 2009, Egamberdieva and Kucharova 2009). In earlier works Lindberg et al. (1985) and Frankenberger and Arshad (1995) suggested that root colonizing bacteria that produce phytohormones, when bound to the seed coat of a developing seedling may act as a mechanism for plant growth stimulation. In response to root exudates, the root associated microorganisms including symbiotic species within the genera Rhizobium synthesize indoleacetic acid (IAA) (Costacurta and Vanderleyden 1995; Frankenberger and Arshad 1995; Mehnaz and Lazarovits 2006). Tsavkelova and others (2007) studied interactions of rhizobacteria with orchid Dendrobium moschatum via indole-3-acetic acid (IAA) and they found that bacterial strains which belong to genera such as Rhizobium, Microbacterium, and Mycobacterium were among the most active IAA producers. Plant cells take up some of the IAA that is secreted by the bacteria and, together with the endogenous plant IAA, can stimulate plant cell proliferation (Glick et al. 2007). Earlier reports showed that Rhizobium meliloti associated with alfalfa, produced 20 µg/ml of IAA (Williams and Singer 1990), whereas Rhizobium leguminosarum produced 2.0 µg/ml of IAA (Beltra et al. 1980). IAA produced by nodule bacteria is transported to other parts of the plant and might be involved in several stages of the symbiotic relationship (Wheeler et al. 1979; Hunter 1989; Badenoch-Jones et al. 1983). Lindow et al. (1998) studied IAA producing bacteria associated with pear trees and found relatively high percentage of epiphytic bacteria on pear leaf and fruit surfaces. They were belonging to different genera such as Pantoea agglomerans, Pseudomonas syringae, P. viridiflava, P. fluorescens, P. putida, and Rahnella aquaticus. Barea et al. (1976), found that 90% rhizosphere bacteria isolated from various Indole-Acetic Acid Production by Root Associated Bacteria … 5 plants were able to produce IAA. In other studies also reported that most of Pseudomonas strains produced auxin like compounds (Ataei 2005; Zadeh 2006; Khakipaour et al. 2008). Ruanpanun et al. (2010) found high IAA producing nematophagous actinomycete and fungal isolates such as Aspergillus sp., Streptomyces sp. Tsavkelova et al. (2006) observed IAA production in fungi in genera Aspergilllus sp., Fusarium sp. and Paecilomyces sp. The synthesis of auxin by rhizosphere bacteria often depends on the presence of phytohormone precursor tryptophan in the root exudates of plants. The presence of Ltryptophan in the medium is a necessary condition of the biosynthesis of indole-3-acetic acid (IAA) by rhizosphere bacteria (Kravchenko et al. 2004; Kamilova et al. 2006). The rhizobacteria possesses plant growth–promoting activity when the concentration of tryptophan in the rhizosphere is sufficiently high (Kravchenko et al. 2004). Rhizosphere strains consuming of root exudates including available Trp and it is converted to IAA, whereas plant and bacteria may regulate the IAA biosynthesis in the rhizosphere (Dakora and Philips 2002). In earlier studies Skerman (1959) observed when the bacteria were grown in tryptophan supplemented yeast extract mannitol medium, they produced high amount of IAA. Ahmad et al. (2005) also reported that with an increase in tryptophan concentration from 1 to 5 mg/ml, the production of IAA in fluorescent Pseudomonas isolates increased. Other studies also reported that Trp considerably stimulates microbial IAA yield in vitro (Ivanova et al. 2001; Khalid et al. 2004; Egamberdieva and Kucharova 2009). We have also observed that plant growth promoting bacteria isolated from the rhizosphere of wheat produced IAA, where Trp increased its biosynthesis (Table 2). Table 2 The production of IAA by plant growth promoting, root colonizing bacteria (in medium with tryptophan and without tryptophan). Bacterial test strain IAA production without tryptophan Pseudomonas putida TSAU1 5,3 Pseudomonas extremorientalis TSAU6 5,7 Enterobacter hormaechei TSAU2 17,6 Psedomonas extremorientalis TSAU20 5,7 Pantoea agglomerans TSAU16 10,5 Pseudomonas `chlororaphis TSAU13 4,3 Alcaligenes faecalis TSAU3 7 Pseudomonas aureantiaca TSAU22 9,2 * Auxin (IAA) level in µg/ml after 5 days of incubation with tryptophan 100 µg/ml 23,7 4,8 55,6 7,4 23,1 9,4 8,3 41,3 According Ghosh and Basu (2006) among the three different isomers of tryptophan, the bacteria produced higher amount of IAA with the supplementation of L-tryptophan (138 µg/ml) than in D-tryptophan (15 µg/ml) or DL-tryptophan (84 µg/ml). In earlier work Dullaart (1970) explained this process due to the utilisation of this essential amino acid partly in protein synthesis and partly for the formation of other indole compounds in addition to IAA. Malhotra and Srivastava (2008) studied IAA biosynthesis by A. brasilense under various stress conditions and they found that IAA biosynthesis occurs in a Trp dependent manner, 6 Egamberdieva Dilfuza where exogenous supply of Trp enables the cells to maintain an elevated Trp pool, which is utilized for IAA biosynthesis. It has been found that environmental factors affect IAA biosynthesis by rhizosphere bacteria (Bashan 1998). Ona et al. (2005) reported that oxygen limitation has negative influence on IAA biosynthesis by A. brasilense. In our study we found that salinity may inhibit IAA production of root associated bacteria. However salt tolerant bacterial strain appeared to produce IAA in media with or without tryptophan in saline condition (3%NaCl). The presence of tryptophan stimulated auxin production of bacterial strains (Figure 1). Figure 1. The effect of salinity on IAA production ability of P. aureantiaca TSAU22 (bacteria were grown 7 days in KB medium at 28oC with the absence and presence of 100 µg/ml of the auxin precursor tryptophan). Similar findings reported by Malhotra and Srivastava (2008), where A. brasilense SM was able to withstand the harsh conditions and it could even trigger its IAA synthesizing ability. Thus nutrient stress affected cellular viability of bacterial strain and significantly influenced IAA production. IAA PRODUCING BACTERIA IMPROVE PLANT GROWTH IAA producing bacteria stimulate seed germination, increase root formation, plant growth and development (Lugtenberg et al. 2001; Egamberdieva et al. 2010; Shahab et al. 2009). Different plant seedlings respond differently to variable auxin concentrations and type of microorganisms (Sarwar and Frankenberger 1994). The low amount of IAA produced by plant growth promoting rhizobacteria can stimulate the growth of roots, whereas high IAA production by bacterial strains inhibits the root growth (Loper and Schroth. 1986; Patten and Glick 1996). In earlier studies Pilet and Saugy (1985) reported that root elongation is inversely proportional to exogenous IAA concentrations above a threshold of about 10–6 to 10–9 M. Thus higher amount of IAA produced by bacterial strains often inhibitory to the roots (Xie et al., 1996). Also Yasmin et al., (2004) reported that increasing the concentration of tryptophan Indole-Acetic Acid Production by Root Associated Bacteria … 7 from 1 mg/ml to 5 mg/ml resulted in decreased growth in plants. Other studies reported that Pseudomonas and Alcaligenes strains affects auxin levels in the root by decreasing IAA concentration, thus eliminating its negative effects on plants (Leveau and Lindow 2005). Azotobacter paspali significantly increased the dry weight of shoot and roots of several plant species through IAA producing ability (Barea and Brown 1974), Pseudomonas putida produced low level of IAA, but also stimulated the seedling growth of canola (Caron et al. 1995). The plant growth promoting bacteria supply additional IAA into rhizosphere and stimulate growth of lateral roots and root hairs. This increase nutrient absorbing surfaces and may lead to greater rates of nutrient absorption through which plant growth will increase significantly (Hoflich et al. 1995). The inoculation of wheat seedlings with IAA producing Azospirillum brasilense increased the number and length of lateral roots (Barbieri et al. 1986). IAA production by a number of Acetobacter diazotrophicus isolates from sugarcane was confirmed, in addition to a role in nitrogen fixation, this bacterium could promote rooting and improve sugarcane growth by direct effects on metabolitic processes (Somers et al. 2004). We have also observed where IAA producing Pseudomonas strains stimulated root and shoot length and dry weight of wheat grown in gnotobiotic sand system (Table 3). Similar results were reported in some of the previous studies showing that inoculation was found to affect early plant and root development, plant and root dry weight, grain yield and the N-uptake efficiency of plants (Dobbelaere et al. 2002). Table 3. The effect of IAA producing bacterial strains on shoot, root growth and dry matter of wheat grown in gnotobiotic sand system. Treatments Shoot length, cm Control 8.7 Pseudomonas putida TSAU1 12.8* P. extremorientalis TSAU20 11.7* P. `chlororaphis TSAU13 12.5* P. aureantiaca TSAU22 12.2* P. extremorientalis TSAU6 11.8* 7 days old plants, Significantly different at α =0.05 Root length, cm 11.5 13.8* 13.5* 13.6* 14.4* 11.7 Dry weight, g/plant 0.018 0.029* 0.040* 0.037* 0.034* 0.035* Cakmakci et al (2005) observed that in the greenhouse, inoculations with PGPR increased sugar beet root weight by 2.8 – 46.7% depending on the species. Leaf, root and sugar yield were increased by the bacterial inoculation by 15.5 and 20.8 %, respectively. In other studies IAA producing Pseudomonas inoculants significantly increased root dry weight in wheat (Walley and Germida 1997; Egamberdieva and Kucharova 2009), maize (Egamberdieva 2007), yield in sugar beet (Cakmakci et al. 2001), soybean (Cattelan et al. 1999). Inoculations with P. polymyxa which has ability to produce IAA enhanced grain yield of wheat (de Freitas 2000), stimulated the growth of rice (Sudha et al. 1999) and conifer species (Bent et al. 2002). Hasnain and Sabri (1996) showed that inoculation of wheat with Pseudomonas spp. stimulated plant growth by reducing plant uptake of toxic ions and increasing the auxin content. Under drought stress, co-inoculation of bean (Phaseolus vulgaris L.) with Rhizobium tropici and two strains of P. polymyxa resulted in augmented plant height, shoot dry weight and nodule number (Figueiredo et al. 2008). 8 Egamberdieva Dilfuza We have also observed that the exogenous application of indoleacetic acid producing bacteria as inoculants to cotton plants induce resistance to cotton root rot caused by Fusarium oxysporum and Rhizoctonia solani (unpublished data). In conclusion, the beneficial effect of plant growth promoting rhizobacteria on growth of many plants can be partly explained by their ability to produce IAA. The bacterial IAA may be involved in stimulation of root growth, alleviation of salt stress in both shoot and root of plants and also can trigger induced systemic resistance in plants against various disease. REFERENCES Ahmad F., Ahmad, I., Khan, M.S. (2005). Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan 29, 29-34. Turk J Biol 29 (2005) 29-34 Afzal, I., Basra, Sh., Iqbal, A. (2005). The effect pf seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 1(1): 6-14. Hayat, A.S., Hasan, S., Ahmad, A. (2008). A comparative effect of IAA and 4-Cl-IAA on growth, nodulation and nitrogen fixation in Vigna radiata (L.) Wilczek. Acta Physiol Plant. 30, 35–41. Ataei, N. (2005). Isolation and identification of P. fluorescens auxin hormone producers and study their effects on the growth and yield of wheat. M.Sc. Thesis, Al-Zahra University. Barbieri, P., Zanelli, T. & Galli E. (1986). Wheat inoculation with Azospirillum brasilence Sp6 and some mutants altered in nitrogen fixation and indole-3-acetic acid. FEMS Microbiol. Lett. 36, 87-90. Barea, J.M., Brown, M.E. (1974). Effects on plant growth by Azotobacter paspali related to synthesis of plant growth regulating substances. J.Appl. Bacteriol. 37, 583-593. Barea, J.M., Navarro, E., Montoya, E. (1976). Production of plant growth regulators by rhizosphere phosphate-solubilizing bacteria. J. Appl. Bacteriol. 40, 129-134. Badenoch-Jones, J., Rolfe, B.G., Letham, D.S. (1983). Phytohormones, Rhizobium mutants and nodulation in legumes. III. Auxin metabolism in effective and ineffective pea root nodules. Plant Physiol. 73, 347–352. Bartel, B. (1997) Auxin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:5166[CrossRef]. Bashan, Y. (1998). Inoculants of plant growth promoting bacteria for use in agriculture, Biotechnol. Adv. 16, 729–770. Beltra, R., Diaz, F., Fraile, G. (1980). The formation of growth substances by Rhizobium species. Z. Bakteriol. Parasitenkd Infektionskr. Hyg. Abt. 2 135, 617–622. Berg, G., Opelt, K., Zachow, C., Lottmann, J., Götz, M., Costa, R., Smalla, K. (2005). The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microbiol Ecol. 56, 250-261. Bloemberg, G.V., Lugtenberg, B.J. (2001). Molecular basis of plant growth-promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4, 343-350. Bloemberg, G.V., Lugtenberg, B.J.J. (2004). Bacterial biofilms on plants: relevance and phenotypic aspects. In: Ghannoum R, O'Toole GA (eds) Microbial biofilms. ASM Press, Washington, DC, pp. 141-159. Indole-Acetic Acid Production by Root Associated Bacteria … 9 Cattelan, A.J., Hartel, P.G., Fuhrmann, J.J. (1999). Screening of plant growth_promoting rhizobacteria to promote early soybean growth. Soil Sci. Soc. Am. J. 63, 1670–1680. Caron, M., Patten C.L. & Ghosh, S. (1995). Effects of plant growth promoting rhizobacteria Pseudomonas putida GR-122 on the physiology of canolla roots. Plant Growth Reg. Soc. Am. 22nd proceeding, Ed. Green, D.W, July 18-20. Costacurta, A., Vanderleyden, J. (1995). Synthesis of phytohormones by plant associated bacteria. Crit Rev Microbiol 21, 1–18. Dakora, F.D. &. Philips, D.A. (2002). Root exudates as mediators of mineral acquisition in low-nutrient environments, Plant Soil 245, 35–47. Datta, K.S., Varma, S.K., Angrish, R., Kumar, B., Kumari, P. (1998). Alleviation of salt stress by plant growth regulators in Triticum aestivum L. Biol. Plant. 40: 269-275. Davies, P.J. (1995). The plant hormone concept: concentration, sensitivity, and transport. In: Davies PJ (ed) Plant hormones: physiology, biochemistry, and molecular biology. Kluwer Academic Publishers, Dordrecht, pp 13–18. Debez, A., Chaibi, W., Bouzid, S. (2001). Effect du NaCl et de regulatoeurs de croissance sur la germination d’ Atriplex halimus L. Cahiers Agricultures 10, 135-138. Dhingra, B.R., Varghese, T.M. (1985). The effect of growth regulators on the in vitro germination and tube growth of maize (Zea Mays L.) pollen from plants raised under sodium chloride salinity. Nez E Phytol. 100, 563-569. Dobbelaere, S., Croonenborghs, A., Thys, A., Broek, A.V., Vanderleyden, J. (1999). Phytostimulatory effect of A. brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212, 155–164. Egamberdieva, D., Kucharova, Z., Davranov, K., Berg, G., Makarova, N., Azarova, T., Chebotar, V., Tikhonovich, I., Kamilova, F., Validov, Sh. Lugtenberg, B. (2010). Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils. 47:197-205. Egamberdieva, D. (2009). Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol.Plant. 31, 861-864. Egamberdieva, D., Kucharova, Z. (2009). Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol Fertil Soil 45, 563-571. Egamberdiyeva. D. 2007. The growth and nutrient uptake of maize inoculated with plant growth promoting bacteria affected by different soil types. Appl Soil Ecol. 36, 184-189. Fernández-Falcón, M., Borges, A., Borges-Pérez, A. (2003). Induced resstance to Fusarium wilt of banana by exogenous applications of indoleacetic acid. Phytoprotection. 84, 149153. Figueiredo, M.V.B., Burity, H.A., Martınez, C.R., Chanway, C.P. (2008). Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 4, 182 – 188. Frankenberger, J.W.T., Arshad, M. (1995). Microbial synthesis of auxins. In: Frankenberger WT, Arshad M (eds.) Phytohormones in soils. Marcel Dekker Inc, New York, pp. 35–71. Glick, B.R., Karaturov´ıc, D., Newell, P. (1995). A novel procedure for rapid isolation of plant growth-promoting rhizobacteria. Can. J. Microbiol. 41, 533–536. Glick, B.R., Todorovic, B., Czarny, J., Cheng, Z., Duan, J., McConkey, B. (2007). Promotion of Plant Growth by Bacterial ACC Deaminase Critical Rev Plant Sci. 26, 227–242. Gul, B., Khan, M.A., Weber, D.J. (2000). Alleviation salinity and dark-enforced dormancy in Allenrolfea occidentalis seeds under various thermoperiods. Aust. J. Bot. 48, 745–752. 10 Egamberdieva Dilfuza Gruodien, J., Zvironaite, V. (1971). Effect of IAA on growth and synthesis of N compounds in Lucerne. Luk TSR Aukstuja Mosklo Darbai Biologia 17, 77–87. Ghosh, S., Basu, P.S. (2006). Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiol. Res. 161, 362—366. Gravel, V., Antoun, A.H., Tweddell, R.J. (2007). Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur J Plant Pathol. 119, 457–462. Hasnain, S., Sabri, A.N. (1996). Growth stimulation of Triticum aestvum seedlings under Crstress by nonrhizospheric Pseudomonas strains. Abstract Book of 7th Int Symp on Nitrogen Fixation with Non- legumes. – Faisalabad, Pakistan, P. 36. Haas, D., Defago, G. (2005). Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3, 307-319. Höflich, G., Wiehe, W., Hecht-Buchholz, C.H. (1995). Rhizosphere colonization of different growth promoting Pseudomonas and Rhizobium bacteria. Microbiol. Res. 150, 139-147. Hunter, W.J. (1989). Indole-3-acetic acid production by bacteroids from soybean root nodules. Physiol. Plant. 76, 31–36. Itai, C., Richmond, A.E., Vaada, Y. (1968). The role of root cytokinins during water and salinity stress. Israel J. Bot. 17, 187.-195. Ivanova EG, Doronina NV, Trotsenko IuA (2001) Aerobic methylobacteria are capable of synthesizing auxins. Mikrobiologia 70:452–458 Jackson, M. (1997). Hormones from roots as signals for the shoots of stressed plants. Elsevier Trends J. 2, 22–28. Jameson, P.E. (2000). Cytokinins and auxins in plant-pathogen interactions—an overview. Plant Growth Regul 32 369–380. Kabar, K. (1987). Alleviation of salinity stress by plant growth regulators on seed germination. J. Plant. Physiol. 128, 179-183. Kamilova, F., Validov, S., Azarova, T., Mulders, I., Lugtenberg, B. (2005). Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 7: 1809-1817. Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg BJJ (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 19: 250-256. Production of Auxin Hormone by Fluorescent Pseudomonads 1N. Khakipour, 2K. Khavazi, 3H. Mojallali, 3E. Pazira and 2H. Asadirahmani American-Eurasian J. Agric. & Environ. Sci., 4 (6): 687-692, 2008 Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480 Kampert, M., Strzelczyk, E., Pokojska, A. (1975). Production of auxins by bacteria isolated from pine roots (Pinus syivestris L.) Acta Microbiol. Poll. 7: 135-143. Khan, M.A., Weber, D.J. (1986). Factors influencing seed germination in Salicornia pacifica var. utahensis. Am. J. Bot. 73, 1163–1167. Khan, M.A., Gul, B., Weber, D.J. (2001). Seed germination characteristics of Halogeton glomeratus. Can. J. Bot. 79, 1189–1194. Khan, M.A., Gul, B., Darrell, J. (2004). Weber Action of plant growth regulators and salinity on seed germination of Ceratoides lanata Can. J. Bot. 82, 37–42. Indole-Acetic Acid Production by Root Associated Bacteria … 11 Kloepper, J.W., Lifshitz, R. & Zablotowicz, R. M. (1989). Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 7, 39–43. Kloepper, J. W., Scher, F. M., Laliberte, M., Tipping, B. (1986). Emergence promoting rhizobacteria: description and implications for agriculture. In: Iron, Siderophores, and Plant Disease. pp. 155–164. Swinburne, T. R., Ed., Plenum Publishing Corp., New York. Kravchenko, L.V., Azarova, T.S., Makarova, N.M., Tikhonovich, I.A. (2004). The Effect of Tryptophan Present in Plant Root Exudates on the Phytostimulating Activity of Rhizobacteria Microbiology, 73, 2, 156–158. Lindberg, T., Granhall, U., Tomenius, H. (1985). Infectivity and acetylene reduction of diazotrophic rhizosphere bacteria in wheat (Triticum aestivum) seedlings under gnotobiotic conditions. Biol. Fertile Soil. 1, 123-129. Le Floch, G., Rey, P., De´niel, F., Benhamou, N, Picard, K., Tirilly, Y. (2003). Enhancement of development and induction of resistance in tomato plants by the antagonist, Pythium oligandrum. Agronomie, 23, 455–460. Leveau JH, Lindow S E (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371 Lindow, S.E., Desurmont, C., Elkins, R., McGourty, G., Clark, E., Brandl, M.T. (1998). Occurrence of Indole-3-Acetic Acid–Producing Bacteria on Pear Trees and Their Association with Fruit Russet. Phytopathology, 88, 111, 149-1157. Lifshitz, R., Kloepper, J.W., Kozlowski, M., Simonson, C., Carlson, J., Tipping, E.M., Zalesk, I. (1987). Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can J Microb 33, 390-395. Loper, J. E., Schroth, M. N. (1986). Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 76, 386-389. Lugtenberg, B.J.J., Kravchenko, L.V., Simons, M. (1999). Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ. Microbiol. 1, 439-446. Lugtenberg, B.J.J., Dekkers, L., Bloemberg, G.V. (2001). Molecular determinants of rhizosphere colonization by Pseudomanas. Annu Rev. Phytopath. 39, 461-490. Lugtenberg, B.J.J., Kamilova, F.D. (2004). Rhizosphere management: microbial manipulation for biocontrol. In Goodman RM (eds) Encyclopedia of plant and crop science. Marcel Dekker, New York, pp. 1098-1101. Lugtenberg B, Kamilova F (2009). Plant growth-promoting rhizobacteria. Annu Rev Microbiol (2009) Ann. Rev. Microbiol. 63, 541-556. Macdonald H (1997) Auxin perception and signal transduction. Physiol Plant 100:423–430 Malhotra, M., Srivastava, S. (2008). Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Europ. J. Soil Biol. 1-8. Marschner, P., Yang, CH., Lieberei, R., Crowley, D.E. (2001). Soil plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem. 33, 1437-1445. Mayak, S., Tirosh, T., Glick, B.R. (2004). Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 166, 525–530. Mehnaz, S., Lazarovits, G. (2006). Inoculation eVects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb Ecol. 51, 326–335. 12 Egamberdieva Dilfuza Ona, J. &. Van, Impe, E., Prinsen, J. (2005). Vanderleyden, Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled, FEMS Microbiol. Lett. 246,125–132. Patten, C. L., Glick, B. R. (1996). Bacterial biosynthesis of indole-3- acetic acid. Can. J. Microbiol. 42, 207-220. Pilet, P. E. & Saugy, M. (1985). Effects of applied and endogenous IAA on maize root growth. Planta. 164, 254-258. Pinton, R., Varanini, Z. & Nannipieri, P. (2001). The Rhizosphere as a site of biological interactions among soil components, plants and microorganisms. In: “The Rhizosphere: biochemistry and organic substances at the soil-plant interface”. New York, Marcel and Dekker, Inc. Prakash, L., Parthapasenan, G. (1990) . Interactive effect of NaCl salinity and gibberelic acid on shoot growth, content of absisic acid and gibberelin like substances and yield of rice (Oruza sativa) Plant Science 100, 173-181. Raaijmakers, J.M., Paulitz, C.T., Steinberg, C., Alabouvette, C., Moenne-Loccoz, Y. (2009). The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321, 341-361. Rey, P., Leucart, S., De´silets, H., Be´langer, R. R., Larue, J. P., & Tirilly, Y. (2001). Production of indole-3-acetic acid and tryptophol by Pythium ultimum and Pythium group F: possible role in pathogenesis. European J Plant Path. 107, 895–904. Ruanpanun, P., Tangchitsomkid, N., Hyde, K.D., Lumyong, S. (2010). Actinomycetes and fungi isolated from plant-parasitic nematode infested soils: screening of the effective biocontrol potential, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol. 26, 1569–1578. Saimbhi, M.S. (1993). Growth regulators on vegetable crops. In: K. L. Chadha and G. Kallo (eds.). Advances in Horticulture, vol. 6 (I). Malhotra Publishing House, New Delhi, India. pp 619-642. Sarwar, M. & Frankenberger, W.T. (1994). Tryptophan dependent biosynthesis of auxins in soil. Plant Soil. 160, 97-104. Sarwar, M. and R..J. Kremer, 1995. Determination of bacterially derived auxins using a micro plate method. Lett. Appl. Microbiol. 147: 282-285. Shahab, S. Nuzhat, A., Nasreen, S. K. (2009). Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. African J Agricul Res. 4, 1312-1316. Sakhabutdinova, A.R., Fatkhutdinova, D.R., Bezrukova, M.V., Shakirova, F.M. (2003). Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 314–319. Sharaf, E.F., Farrag, A.A.(2004). Induced resistance in tomato plants by IAA against Fusarium oxysporum lycopersici. Polish J. Microb. 53, 2, 111-116. Singh, H., Dara, B.L. (1971). Influence of presoaking of seeds with gibberellin and auxins on growth and yield attributes of wheat (Triticum aestivum L.) under high salinity, sodium adsorption ratio and boron levels. Indian J. Agr. Sci. 41, 998-1003. Skerman, V.B.D., 1959. A Guide to the Identification of the Genera of Bacteria with Methods and Digests of Generic Characteristics. The Williams and Wilkins Company, Baltimore 2, USA. Indole-Acetic Acid Production by Root Associated Bacteria … 13 Spaepen, S., Vanderleyden, J., Okon, Y. (2009). Plant Growth-promoting actions of rhizobacteria. In: van Loon LC, Ed Kader JC, Delseny M (eds) Adv Botanical Res. 51, 283-320. Srinivasan, P.S., Gopal, K.S. (1977). Effect of plantofix and NAA formulation on groundnut var TMU-7. Curr Sci. 46, 119–120. Somers, E., Vanderleyden, J., Srinivasan, M. (2004). Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev. Microbiol. 30, 4, 205-400. Thomashow, L.S., Weller, D.M. (1996). Current concepts in the use of introduced bacteria for biological disease control: Mechanisms and antifungal metabolites. In: Stacey G, Keen NT (eds) Plant-Microbe Interactions, Chapman and Hall, London, pp 187–235. Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology 2006;7:847-859. Tsavkelova, E.A., Cherdyntseva, T.A., Klimova, S., Shestakov, A.I., Botina, S.G., Netrusov, A.I. (2007). Orchid-associated bacteria produce indole-3-acetic acid, promote seed germination, and increase their microbial yield in response to exogenous auxin. Arch Microbiol. 188, 655–664. Tsavkelova, E.A., Klimova, S.Y., Cherdyntseva, T.A., Netrusov, A.I. (2006). Microbial producers of plant growth stimulators and their practical use: a review. Appl. Biochem. Microbiol. 42, 117–126. Yasmin, S., Baker, M.A.R., Malik, K.A., Hafeez, F.Y. (2004). Isolation, characterization and beneficial effects of rice associated plant growth promoting bacteria from Zanzibar soils. J. Basic Microbiol. 44, 3, 241-252. Xie, H., Pasternak, J. J., Glick, B. R. (1996). Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr. Microbiol. 32:67-71. Van Loon, L.C. (2007). Plant responses to plant growth-promoting bacteria. Eur. J. Plant Pathol. 119, 243-254. Wheeler, C.T., Henson, I.E., Mc. Laughlin, M.E. (1979). Hormones in plants bearing actinomycete nodules. Bot. Gaz. 140 (Suppl.), S52–S57. Williams, M.N.V., Singer, E.R., 1990. Metabolism of tryptophan and tryptophan analogs by Rhizobium meliloti. Plant Physiol. 92, 1009–1013. Woodward, A.W., Bartel, B. (2005). Auxin: regulation, action and interaction. Ann. Bot. 95, 707–735. Zadeh, A.P. (2006). Isolation and identification of P. fluorescens plant growth promoters and study their effects on the growth and yield of canola. M.Sc. Thesis, Tehran University. Zholkevich, V.N., Pustovoytova, T.N. (1993). The role of Cucumis sativum L leaves and content of phytohormones under soil drought. Russ. J. Plant Physiol. 40, 676–680.