tpj13032-sup-0010-MethodsS1
advertisement

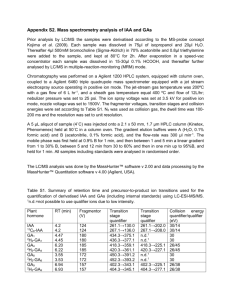
Methods S1 Standards for the IAA were purchased from Wako Chemicals (Osaka) and standards for IPyA were purchased from Sigma-Aldrich Co. LLC. The internal standard of [2H5]-IAA was purchased from Cambridge Isotope Laboratories (MA). To analyze endogenous IAA and IPyA in plants, plant samples were frozen in liquid N2 and homogenized. For IAA analyses, [2H5]-IAA (40 pg/mg fresh weight) were added as an internal standard and dissolved in 800 L of 50% acetonitrile at 4ºC. A mixture of NaCl (20 mg), MgSO4 anhydrous (80 mg), sodium citrate tribasic dehydrate (20 mg) and sodium dihydrogencitrate (10 mg) was added and partitioned with voltex and centrifugation. Supernatant of the acetonitrile layer (app. 400 μL) was adjusted to 80% acetonitrile by adding 100 L of MilliQ water and subjected to an OASIS HLB SPE cartridge column (1 cc, 30 mg, Waters), and eluted with 500 L of 80% methanol (MeOH). The elutes were combined and evaporated to dryness in vacuo and dissolved with MilliQ water (500 L), then subjected to an OASIS MCX SPE cartridge and eluted with 500 l of MeOH to produce an IAA fraction. For IPyA analyses, after the partition without adding the internal standard of IAA, supernatant of the acetonitrile layer (app. 400 μL) was placed on ice, 400 μL of MeOH and 100 μL of methoxyamine-HCl in pyridine (contains 2%) were added and incubated for 30 min at 60ºC to metoximation. The reaction mixture was evaporated to dryness in vacuo, dissolved with MilliQ water (500 μL), and subjected to the OASIS HLB and OASIS MCX SPE cartridge columns with the same process as described above to produce the IPyA-methoxime fraction. The IAA and IPyA-methoxime fractions were analyzed by UPLC-ESI-MS/MS (ACQUITY Ultra-Performance Liquid ChromatographyTQ Detector, Waters) with multiple reaction monitoring (MRM) mode. Each fraction was evaporated to dryness in vacuo, dissolved with 5% acetonitrile (90 L), and 50 l was injected into an ACQUITY UPLC BEH C18 Column (1.8 mm, 2.1 100 mm; Waters) connected with an ACQUITY UPLC BEH 1 C18 VanGuard Pre-Column (1.7 mm, 2.1 5 mm; Waters). The IAA fraction were eluted using a 24-min gradient consisting of 0.05% formic acid in water (A) and 0.05% formic acid in MeOH (B), at a flow rate 0.2 mL/min and column temperature of 35ºC. The following gradient was carried out with 5 of B (0 to 5 min), 5 to 55% of B (5 to 18 min), 55 to 100% of B (18 to 20 min), 100%of B (20-24min), following the end of the gradient re-equilibrated to initial conditions (10 min). The IPyA-methoxime fraction were eluted using a 15-min gradient consisting of 0.05% formic acid in water (A) and 0.05% formic acid in MeOH (B), at a flow rate 0.1 mL/min and column temperature of 35ºC. The following gradient was carried out with 5 to 85% of B (0 to 8 min), 85 to 100% of B (8 to 9 min), 100%of B (9-15min), following the end of the gradient re-equilibrated to initial conditions (10 min). The effluent was introduced into the TQ Detector (Waters) with the optimal settings as follows: capillary voltage 3 kV, extractor voltage 3 V, RF lens 0.1 V; source temperature 150ºC, desolvation temperature 350ºC; desolvation gas flow 600 L/h, cone gas flow 50 L/h. Quantification of IAA was performed by MRM of the precursor and the appropriate product ion using the optimal fragmentor voltage (30V) and collision energy (21V), determined using MassLynx™ Quan Optimize software version 4.0 (Waters) for the various diagnostics transitions. Diagnostic MRM transitions obtained by the precursor and the appropriate product ion and retention times for quantification of IAA were (IAA; 176.1 > 130.1: Rt. 13.753 ± 0.005 min, [2H5]-IAA; 181.2 > 134.1: Rt. 13.768 ± 0.005 min, means ± SD; n=6). Chromatograms were analyzed using the QuanLynx™ software version 4.0 (Waters), and the compounds were quantified by a standard curve from the ion peak area ratios of standards versus internal standards (IAA/[2H5]-IAA [12.5500 pg/500 pg, R2=0.9999]). Comparisons of IPyA relative contents were calculated from the ratio of MRM transition intensity obtained by the precursor and the appropriate product ion (IPyA-methoxime, m/z: 233.1 > 130.1) using optimal fragmentor voltage (20V) and collision energy (20V) determined using the MassLynxTM Quan Optimize software version 4.1 (Waters). The retention time of IPyA- 2 methoxime was 9.020 ± 0.000 min (means ± SD; n=6). 3