Activities Near-field Optical Microscopy Investigation of Optical
advertisement

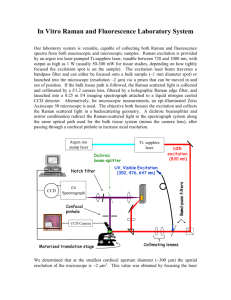
ACTIVITIES NEAR-FIELD OPTICAL MICROSCOPY INVESTIGATION OF OPTICAL INTERACTION AT THE NANOSCALE REU Student: Rachel Chapla Graduate Student Mentor: Karissa I. Kiesow Faculty Mentor: Dr. Terefe G. Habteyes A. Introduction The excitation of metallic nanoparticles has several applications, most of which are centered around biomolecule sensing. Outside the field of biomedicine, metallic nanoparticle excitations can be used in light harvesting and optoelectronics, where light energy is transmitted through a chain or an array [1,2,3]. This excitation refers to the coupling of the conductive particles’ dipole moments to the oscillations of an excitation source in order to induce an evanescent electric field, or near-field signal. Once the use of scattering near-field optical microscopy was demonstrated to be the optimal method for detecting and measuring the phase and amplitude of the near-field produced by the nanoparticle excitation, studies were performed to determine the effects of cross polarization, particle size, tip composition and location, and distance between particles on near-field detection, as well as how different excitation modes can be attained [4,5,6,7]. This study followed the aforementioned path of research by investigating the excitation of closely spaced gold nanorods positioned at varying gap lengths, while also forging an unproven relationship between the gap mode excitation from the coupling of these nanorods with the Raman signal of analyte molecules placed in the gap, which are used for determining the effect of outside factors on Raman signal. In a study by Alonso-González et al., it was discovered that light intensity elastically scattered by an object located in the hot spot of a metal nanoparticle increases greatly upon excitation of the nanoparticle; however, this study did not utilize a Raman analyte molecule, but rather mimicked one with an oscillating Si tip. The scattering performed by this tip was elastic, but the mechanism was the same as that of Raman scattering, so the study speculated that there is a correlation between Raman signal enhancement and near-field excitation in hot spots [8]. This study sought to strengthen this argument by investigating the Raman signal of analyte molecules in the hot spot created by a strong near-field between two coupled gold nanostructures. The method implemented in this study was similar to past studies that determined that there is a clear Raman signal enhancement when a Raman analyte molecule is placed in close proximity to an excited metallic nanoparticle, but while these studies have correlated the Raman intensity with the fourth power of the metallic structures’ field enhancement, none have demonstrated that the Raman enhancement takes place in the same location as the near-field signal [8,9]. B. Background B1. aNSOM Imaging: Excitation and Modes The apertureless near-field scanning optical microscope, or aNSOM, provides images of the topography and near-field intensity and, when customized for pseudoheterodyne interferometric detection, phase data, of a sample by exciting the sample with a laser beam aligned at an Atomic Force Microscopy (AFM) or Scanning Tunneling Microscope (STM) probe tip. When the tip and sample are within a very small distance of each other, they couple. Both the tip and the sample become excited when the excitation beam is focused on the tip-sample junction, and the strength of the tip-sample coupling mechanism is dependent on these excitations. Sample excitation occurs when the excitation source, in this case the laser beam, is in resonance with the sample, meaning that the oscillation of the beam matches the dipole moment of the sample. At resonance, the polarizability of the sample is at its peak, allowing the beam to couple to this dipole moment, and the plasmons, or collective oscillations of electrons, in the sample begin to oscillate in phase with the oscillation of the beam. The electric field producing these oscillations is known as the near-field, and it is an evanescent field; thus, its strength decreases exponentially with increasing distance from the surface. The tip, as well as being excited in the same manner, serves to scatter the near-field that is formed so that it is measureable [10]. Because the sample consists of collections of oscillating charge, different modes are formed, including gap mode, dipolar mode, quadrupolar mode, and sometimes even higher order modes. When these excited areas contain a large amount of concentrated charge, they are often referred to as “hot spots” [4]. Gap mode, which is slightly different from the others, occurs when the region of excitement is in a gap between two closely spaced particles. These particles are said to be coupled, and they act as a dimer rather than two separate particles. In a study by Kim et al., it was found that sets of 2 gold nanocubes with an average gap distance of 4 nm were capacitively coupled. The proof of the coupling was the presence of a hot spot in the gap between the two cubes (as opposed to at the edges of the cubes, which is an indicative characteristic of uncoupled nanostructures) recorded on the aNSOM S/S-polarization setting [10]. Both the excitation and the detection light can be polarized either in the S- or P-direction. Spolarized light consists of waves oscillating parallel to the sample plane, and P-polarized light refers to waves oscillating perpendicularly to the sample plane, meaning they are in-plane with the tip. An S/P-polarized scan means that the sample is excited with S-polarized light and the resulting scattered light is P-polarized before detection. The excitation and detection polarizations are influential on the results because they help determine the excitation and scattering efficiency as well as the strength of the tip-sample interactions and the interactions between different structures within the sample. The S/S-polarization setting is often used to find the near-field signal for coupled gold nanostructures because it provides strong in-plane excitation and interaction due to the incident beam being polarized along the plane in which the sample plasmons are oriented. Although it was once believed that the tip was not involved in S/S-polarized scanning, Kim and Kim’s reports of significant in-plane polarizability of metalcoated tips contradicted this notion, and Mastel et al.’s claim that the hot spot detected with S/Spolarization in the gap between gold antennas was caused by the platinum tip loading the nanogap and creating a charge buildup supported the findings of Kim and Kim that the tip had a significant role in S/S-polarization [4,11]. P-polarized excitation is not often used when investigating the coupling of metallic nanostructures because, while it provides for strong tipsample coupling, this polarization of excitation creates too much tip influence on the near-field signal. B2. Raman Spectroscopy Raman spectroscopy is a method used for detection and characterization of molecules by means of measuring the inelastic scattering of excitation light by a sample. Inelastic scattering occurs when energy is not conserved, causing photons to shift in frequency in order to return to their ground energy state [12]. Multiple methods are utilized to enhance a Raman signal, which is, unaided, typically on the order of 10-30cm2 for a single molecule, a strength that is very difficult to detect [9]. One method, known as Surface-enhanced Raman Scattering, or SERS, utilizes the interaction of an analyte molecule with a metallic structure. The enhancement of the Raman signal can be attributed to the increased optical fields created by the excitation of the metallic structure as well as to the chemical effect of a charge transfer between the metal and the analyte molecule [13,14]. The transfer of charge from the metal particle to the analyte molecule results in an increase in the polarizability of the molecule [15]. It was found in a study by Nie and Emory, et al., which utilized rhodamine 6G adsorbed on colloidal gold, that the Raman signal could be increased to a magnitude on the order of 10-15cm2, showing an enhancement of 1015 power [9]. Another study by Kneipp, et al., that used colloidal silver as a substrate and crystal violet as an adsorbate, supported the aforementioned study by obtaining a magnitude enhancement by a factor of 1014 [13]. Another method of Raman signal enhancement known as Tip-enhanced Raman spectroscopy, or TERS, physically separates the electromagnetic and chemical enhancement mechanisms. In this method, which has been most successful when analyte molecules are adsorbed on a smooth metallic surface, the chemical enhancement is due to charge transfer from the metallic surface to the adsorbate (the same as in SERS), while the electromagnetic field enhancement is highly confined because it is caused by a probing tip [14]. The tip is positioned in such close proximity to the sample that it creates an optical cavity, allowing for the excitation of the sample’s localized surface plasmons with an incident illumination [16]. In both TERS and SERS, the metallic particle or surface also serves to quench fluorescence, or decrease the fluorescence by means of charge transfer. (Fluorescence is a resonant emission, which contrasts with Raman’s inelastic scattering.) The two enhancement methods were used in combination in another study by Pettinger, where the tip was found to be responsible for the electromagnetic enhancement, while the metallic substrate provided chemical enhancement via charge transfer. Although this enhancement mechanism is very similar to that of TERS, the metallic substrate was slightly roughened in order to invoke the SERS effect. This method, which was utilized in this study, is advantageous because it allows for control of the distance between the tip and the sample and it provides an accurate Raman signal due to effective fluorescence quenching. Also, the metal substrate has a strong near-field due to the tip-sample coupling [14]. C. Research Objective: The research objective of this study was to locate a near-field signal indicating gap mode excitation that is characteristic of the capacitive coupling between gold nanorods and to show the relationship between near-field signal from this gap mode excitation and enhanced Raman signal of an analyte molecule located in the nanorod gap. This goal was to be accomplished by creating a sample with coupled gold nanorods, investigating the gap excitation of the coupled nanostructures, and then placing analyte molecules in the gap and characterizing these molecules to determine if the excitation would cause an increase in Raman signal. D. Methodology While most of the past studies investigating Raman enhancement used Au or Ag aggregates as substrates, a study by Nie and Emory mentioned the possibility of increasing control of nanoparticle shape and size by using highly ordered vapor-deposited metallic nanostructures [9]. Because electron beam deposition technology was accessible, this notion was followed and gold nanorods were deposited onto a functionalized substrate. The sample fabrication process was a 3-step procedure consisting of electron beam lithography, electron beam metal deposition, and liftoff, which together created gold nanostructures in a preferred pattern on a substrate. The substrate, in this case SiO2, was covered in a layer of Poly(methyl methacrylate), PMMA, and a conductive polymer known as aquaSAVE, and the desired design, rows of coupled nanorods with varying gap lengths, as shown in Figure 1, was etched into the PMMA layer using an electron beam. The etching was accomplished because the PMMA acted as a resist film that was sensitive to the electrons in the beam, and as the beam scanned the film, its energy removed the PMMA in designated areas [17]. This process is known as electron beam lithography, the beam used in this study had 100keV of energy, 500pA of beam current, and a dose of roughly 2000μC/cm2. The electron beam deposition was performed by Vistec VB300 e-beam lithography system at The Molecular Foundry at the University of California, Berkley. The aquaSAVE and part of the PMMA were then removed with water and sonication for 100 seconds in a 7:3 mixture of isopropyl alcohol and water [18]. At this point, the deposition procedure was ready to begin. Electron beam depositions were performed at the Center for Integrated Nanotechnologies Core Facility (CINT). The sample was placed in a chamber and an electron beam heated the deposition metal so that it vaporized and then precipitated, depositing on all surfaces in the chamber at a controlled rate to form a layer of a specified height. The sample deposition consisted of a layer of Ti followed by a layer of Au. Although Ti is known to damp the near-field signal of Au, it was deposited beforehand in order to functionalize the SiO2 substrate, making possible the adhesion of Au. Then, because the whole sample was covered in titanium and gold, a liftoff procedure was required. The liftoff procedure consisted of soaking the sample in multiple acetone baths and rinsing it with an acetone airbrush at 25 psi. The liftoff served to remove not only the unwanted gold and titanium from the surface of the remaining PMMA layer, but also the PMMA film itself, so that all that remained on the substrate were the titanium and gold layers that were deposited into the etched cavities. Two samples were fabricated, one consisting of a 2 nm Ti layer and a 24 nm Au layer (Sample A) and a second consisting of a 1 nm Ti layer and a 25 nm Au layer (Sample B). (A) (B) Figure 1. Gold nanorod design. A.) A large view of the design: The gold triangles and letter E serve as gold markers for finding the samples with a microscope and for performing initial scans to ensure that the microscope is calibrated and aligned to provide a strong topographical image and near-field signal. The nanorods are in pairs, and the gap distance differs from section to section as labeled. A closer view of one of the unit cells that make up each of the sections is depicted in B.). Within each section, all pairs have the same gap distance and all rods have a width of 40nm and a height of 25nm, but the rod length increases incrementally from 40nm to 100nm. Many of these unit cells are contained in each section. The first test to perform after fabricating the sample was the aNSOM near-field scans to determine the locations of excitation on the sample. The amplitude and phase of the near-field signal of the samples were measured using the neaSNOM aNSOM by Neaspec with pseuduheterodyne interferometric detection, as seen in Figure 2. This microscope works in conjunction with a 632.8 nm Thor Labs He-Ne laser beam to excite the sample and record the induced near-field. The laser first passes through a half-waveplate, an instrument that allows for the selection of either S- or P-polarized incident light. The first polarizer then refines the beam even further to this selected polarization. When the beam reaches the beam splitter, it is divided into two equal parts, the excitation beam and the reference beam. The excitation beam is reflected by first a plane mirror and then a parabolic mirror (NA =0.46), which can be adjusted to focus it onto the tip-sample junction. The tip, a Pt/Ir coated Si AFM probing head with a radius of curvature of 25 nm, provides both a near-field signal and a topographic image of the sample. In order to create a topographic image, the tip scans the sample surface while tapping it at a tapping amplitude of ~50 nm and at a constant oscillating frequency of around 240 kHz [6]. To maintain this frequency, the tapping force, controlled by the voltage, must be adjusted. These adjustments in force are monitored and used to image the sample’s topography. The near-field is scattered by the tip back to the parabolic mirror, to the plane mirror, and to the beam splitter, where it is recombined with the reference beam by the interferometer. Within the interferometer, the reference beam has been directed through a quarter waveplate, allowing for the selection of S- or P-polarization before recombination. Combining the scattered light with the reference beam causes the scattered light to take on the selected polarization, and thus, phase, of the reference beam. Together, the beams are directed through a second polarizer, which again refines the chosen polarization, and focused by a lens onto a Silicon New Focus photodiode detector (Model 2051.) Pseudoheterodyne detection is implemented, meaning the far-field, or light from extraneous sources, is filtered out in 4 harmonic demodulations. As the harmonic demodulation number increases, more of the far-field is filtered out and the near-field, although it appears to be weaker, is more accurate. Because demodulation suppresses the farfield and the reference beam strengthens the near-field of the scattered light, provides phase data, and clears the beam of extraneous light sources, background-free detection is accomplished. The scattered light is also directed by the parabolic mirror to a different plane mirror and then through a long pass filter, which filters out a specific range of wavelengths. For this experiment, its function is to eliminate the excitation wavelength so that only the scattered emission, will pass through to the spectrometer or Avalanche Photo Diode. The flipper mirror allows the user to choose to which of the two characterization instruments they will send the scattered light, and lenses focus the light on each instrument. Figure 2. The Neaspec aNSOM As a cutting-edge microscope, the aNSOM requires an involved procedure to calibrate before each use. After installing a new tip, the knife’s edge, an internal mechanical device, must be calibrated and the lasers must be aligned using an overlay image of the tip and a “dummy” sample. Once these alignments are made, the real sample must be placed at a 90° angle to the kvector, or propagation vector of the excitation beam. The reason for this specific placement, shown in Figure 3, is that electric field induced, | |, is perpendicular to the k-vector, either inplane with the sample (s-polarized) or in-plane with the tip (p-polarized). Dipole moments form in the gold nanorods when they are placed together, and aligning the rods so that their dipole moments are parallel to the s-polarized electric field creates the largest in-plane Hamiltonian energy (H), according to the equation where is the dipole moment of a gold nanorod, and is the angle between the electric field and the dipole moment. The sample must be focused very slowly and carefully so as to not break the tip, which is so delicate that any unintentional contact will break it off. Then the sample is moved so the tip is located on a region of gold (often a gold structure that is separated from the sample nanostructures) and, after the excitation beam is aligned at the tip one final time, scans are run for a topographic and near-field signal to ensure that the tip is located on the edge of the gold section. Scans at this location show a sharp contract between the silicon substrate and the gold marker, allowing the user to make adjustments to the laser alignment and the interferometer in order to optimize the near-field signal before scanning the actual sample. The interferometer is manually adjusted so the scattered beam and the reference beam are combined to allow the amplification, uncluttering, and addition of phase data to the scattered beam. Figure 3. Excitation direction of the gold nanorods. The K-vector indicates the direction of the excitation beam. The two possible directions of the electric field are denoted by | (P) and | (S), and they are both perpendicular to the k-vector. The direction of the electric field is determined by the selection of S- or P- excitation polarization. The dipole moments of the rods are shown by the symbols , and they are parallel to the S-polarized electric field. After performing FM scans to determine that there were hot spots in the gaps between the gold nanorods, the analyte molecules were deposited on Sample B. Although past studies used concentrations in the range of 10-13 to 10-4 M solutions of analyte molecules, A solution was created to be as concentrated as possible without becoming super-saturated [19,13,20]. The reason for the incongruity in molarities is that while past studies aimed to enhance Raman signals to the point of optimal single-molecule detection, the goal of this study was to demonstrate a correlation between gap excitation and Raman signal enhancement. Therefore, the highest possible analyte molecule concentration was desired so that as many adsorbates as possible would take up residence in the nanorod gaps and would help show an enhanced Raman signal, regardless of the number of molecules. The three adsorbates chosen were 1,2-Di(4pyridyl)ethylene, or BPE, best known for its profilic role in the 1995 study by Kneipp et al., in which the Raman signal of BPE was significantly increased by drop-coating it onto a TEM grid containing colloidal Au, Crystal Violet, used in past SERS studies where it experienced a range of enhancements by a factor of 106 to 1014, and Rhodamine 6G, which was proven to have a SERS enhancement by a factor of 1014 to 1015 when reacting with a silver colloidal solution [13,9,21]. A stock solution of 0.1M BPE in 70% ethyl alcohol was created and then used to make a 5mL solution of 0.01M BPE in 70% ethyl alcohol. Sample B was placed in the 5mL solution for roughly 24 hours in a method known as dip-coating. The beaker was covered for the first 19 hours and then left uncovered after monitoring during the last 5 hours, during which time crystals formed in the solution, many of which adhered to the sample. The sample was rinsed in distilled water and blown dry with nitrogen, neither of which removed all of the crystals. At this time, aNSOM scans were performed again, and Raman scattering information was also gathered. Although the Raman signal is usually measured using a Raman Spectrometer, which provides a spectrum of the Raman intensity of the scanned area, the flipper mirror was adjusted so that the scattered beam was directed to the Avalanche Photo Diode, or APD, to determine the Raman enhancement attributable to the metallic electromagnetic excitement. This instrument works in conjunction with the spectrometer to create a spectrum of either fluorescence or Raman signal, but because the current goal is only to prove the presence and location of a Raman signal enhancement before focusing later on the magnitude of the enhancement, only the APD was used, which provided an image of the illumination of the emission on the screen. The enhancement of the Raman signal is known to be visible as a contrast within this illumination. Gwyddion software was used to analyze the Silicon New Focus Photodiode and APD images and ImageJ software was used to make measurements on the images. After performing all near-field and Raman scans, the imaging of the nanorods in order to measure their lengths and gap distances was performed using SEM technology. Sample A was characterized using the Zeiss Ultra 60 FE SEM at The Molecular Foundry, and Sample B was characterized with the Nova 600 Nanolab High Resolution Scanning Electron Microscope and Focused Ion Beam DualBeamTM machine from FEI Company, located at CINT. This machine, while being operated at 15kV in secondary electron mode, has a resolution of 1.1 nm. E. References [1] A.M. Funston, C. Novo, T.J. Davis, P. Mulvaney, Plasmon Coupling of Gold Nanorods at Short Distances and in Different Geometries, Nano Lett., Vol. 9, No. 4, pp. 1651-1658 (2009). [2] S. Schultz, D.R. Smith, J. Mock, D.A. Schultz, Single-target molecule detection with nonbleaching multicolor optical immunolabels, PNAS, Vol. 97, No. 3, pp.996-1001 (2000). [3] I. Romero, J. Aizpurua, G.W. Bryant, F. J. Garcia de Abajo, Plasmons in nearly touching metallic nanoparticles: singular response in the limit of touching dimers, arXiv preprint (2006). [4] S. Mastel, S. E. Grefe, G. B. Cross, A. Taber, S. Dhuey, S. Cabrini, P. J. Schuck, Y. Abate, Real-space mapping of nanoplasmonic hotspots via optical antenna-gap loading, Applied Physics Letters, Vol. 101, Issue 13 (2012). [5] R. Vogelgesang, J. Dorfmüller, R. Esteban, R.T. Weitz, A. Dmitriev, K. Kern, Plasmonic nanostructures in aperture-less scanning near-field optical microscopy, Phys. Stat. Sol. (b), Vol. 245, No. 10, pp. 2255-2260 (2008). [6] T.G. Habteyes, S. Dhuey, K.I. Kiesow, A. Vold, Probe-sample optical interaction: size and wavelength dependence in localized plasmon near-field imaging, Department of Chemistry and Chemical Biology and Center for High Technology Materials, University of New Mexico (2013). [7] M. Schnell, A. García-Etxarri, J. Alkorta, J. Aizpura, R. Hillenbrand, Phase-Resolved Mapping of the Near-Field Vector and Polarization State in Nanoscale Antenna Gaps, Nano Lett., Vol. 10, No. 9, pp. 3524-3528 (2010). [8] P. Alonso-González, P. Albella, M. Schnell, J. Chen, F. Huth, A. García-Etxarri, F. Casanova, F. Golmar, L. Arzubiaga, L.E. Hueso, J. Aizpurua, R. Hillenbrand, Resolving the electromagnetic mechanism of surface-enhanced light scattering at single hot spots, Nature Communications, Vol. 3, No. 684 (2012). [9] S. Nie, S.R. Emory, Probing Single Molecules and Single Nanoparticles by SurfaceEnhanced Raman Scattering, Science, Vol. 275, pp. 1102-1106 (1997). [10] D.S. Kim, J. Heo, S.H. Ahn, S.W. Han, W.S. Yun, Z.H. Kim, Real-Space Mapping of the Strongly Coupled Plasmons of Nanoparticle Dimers, Nano Lett., Vol. 9, No. 10, pp. 3619-3625 (2009). [11] D.S. Kim, Z.H. Kim, Role of in-plane polarizability of the tip in scattering near-field microscopy of a plasmonic nanoparticle, Optics Express, Vol. 20, No. 8, pp. 8689-8699 (2012). [12] R. Nave, Raman Scattering, HyperPhysics, Department of Physics and Astronomy, Georgia State University, 2013. [13] K. Kneipp, Y.Wang, H. Kneipp, L.T. Perelman, I. Itzkan, R. R. Dasari, M. S. Feld, Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS), Physical Review Letters, Vol. 78, No. 9, pp. 1667-1670 (1997) [14] B. Pettinger, G. Picardi, R. Schuster, G. Ertl, Surface-enhanced and STM-tip-enhanced Raman Spectroscopy at Metal Surfaces, Single Mol., Vol. 5, pp. 285-294 (2002). [15] E. Bailo, V. Deckert, Tip-enhanced Raman Scattering, Chem. Soc. Rev, Vol. 37, Issue 5, pp. 921-930 (2008). [16] B. Pettinger, B. Ren, G. Picardi, R. Schuster, G. Ertl, Nanoscale Probing of Adsorbed Species by Tip-Enhanced Raman Spectroscopy, Physical Review Letters, Vol. 92, No. 9 (2004). [17] Levinson, H. J, M.A. McCord, F. Cerrina, R.D. Allen, J.G. Skinner, A.R. Neureuther, M.C. Peckerar, F.K. Perkins, M. J. Rooks. (1997). P. Rai-Choudhury (Ed.), Handbook of Microlithography, Micromachining, and Microfabrication. (Vol. 1: Microlithography). The Society of Photo-Optical Instrumentation Engineers. [18] T.G. Habteyes, S. Dhuey, E. Wood, D. Gargas, S. Cabrini, J.P. Schuck, A.P. Alivisatos, S.R. Leone, Metallic Adhesion Layer Induced Plasmon Damping and Molecular Linker as a Nondamping Alternative, ACS Nano, Vol. 6, Issue 6, pp.5702-5709 (2012). [19] M. Salemo, G. Das, N. Patra, A. Toma, E. Di Fabrizio, A. Diaspro, Large-Scale Lithography-Free Fabrication of SERS Substrates by Gold Coating of Anodic Porous Alumina, Italian Institute of Technology, Academia.edu [20] A.Górska, S.Meyer, A.Balter, The Silver Colloidal Solutions and Films for Efficient SERS, Acta Physica Polonica A, Vol. 122, No. 2, pp.350-352 (2012). [21] R.G. Freeman, K.C. Grabar, K.J. Allison, R.M. Bright, J.A. Davis, A.P. Guthrie, M.B. Hommer, M.A. Jackson, P.C. Smith, D.G. Walter, M.J. Natan, Self-Assembled Metal Colloid Monolayers: An Approach to SERS Substrates, Science, Vol. 267, pp.1629-1632 (1995).