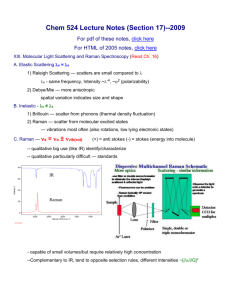
In Vitro Raman and Fluorescence Laboratory System Our laboratory
advertisement

In Vitro Raman and Fluorescence Laboratory System Our laboratory system is versatile, capable of collecting both Raman and fluorescence spectra from both macroscopic and microscopic samples. Raman excitation is provided by an argon ion laser-pumped Ti:sapphire laser, tunable between 720 and 1000 nm, with output as high as 1 W (usually 50-300 mW for tissue studies, depending on how tightly focused the excitation spot is on the sample). The excitation laser beam traverses a bandpass filter and can either be focused onto a bulk sample (~1 mm diameter spot) or launched into the microscope (resolution ~2 m) via a prism that can be moved in and out of position. If the bulk tissue path is followed, the Raman scattered light is collected and collimated by a f/1.2 camera lens, filtered by a holographic Raman edge filter, and launched into a 0.25 m f/4 imaging spectrograph attached to a liquid nitrogen cooled CCD detector. Alternatively, for microscopic measurements, an epi-illuminated Zeiss Axioscope 50 microscope is used. The objective both focuses the excitation and collects the Raman scattered light in a backscattering geometry. A dichroic beamsplitter and mirror combination redirect the Raman-scattered light to the spectrograph system along the same optical path used for the bulk tissue system (minus the camera lens), after passing through a confocal pinhole to increase axial resolution. Argon ion pump laser Ti: sapphire laser Dichroic beam-splitter CCD f/4 Spectrograph Confocal pinhole UV, Visible Excitation (352, 476, 647 nm) filter Band-pass filters Notch filter NIR excitation (830 nm) CCD Camera Motorized translation stage Collimating lenses We determined that at the smallest confocal aperture diameter (~300 m) the spatial resolution of the microscope is ~2 m3. This value was obtained by focusing the laser spot onto a single, 1 m diameter polystyrene bead. Moving the sample stage laterally by 1 m caused a signal decrease of about 90 %. Axially, we determined the signal to decrease that much after about 2 m translation. This resolution is comparable to what has been described for other confocal Raman systems. In practice, the lateral resolution obtained from tissue samples is approximately 2 m. This sampling volume is smaller than the dimensions of all morphological features of interest for developing a spectroscopic model (typical cell diameter ~10 m). The spectrograph itself has an adjustable slit and a turret, which holds three gratings for a range of measurements. For the Raman studies a 600 grooves/mm grating blazed at 1 m is used along with the 140 m spectrograph entrance slit setting, providing ~ 8 cm-1 resolution. As most biological samples do not exhibit Raman bandwidths narrower than 10 cm-1, a 140 m spectrograph entrance slit makes sense, providing increased optical throughput. A CCD camera atop the microscope allows for registration of the focused laser spot with a white light trans-illuminated image. The microscope itself is equipped with a range of objectives, both normal and phase contrast. Typically, for Raman studies we used a 63x infinity-corrected water immersion objective (0.9 NA). As both the detector and the microscope translation stage are computer controlled, spectral maps of the tissue can be created by moving the translation stage in a raster-scan pattern under the microscope objective. Simply by changing the excitation source, the filters/beamsplitters, and spectrograph grating, the system can be adapted for collecting fluorescence measurements. Three additional wavelengths, 352 (UV argon), 477 (argon), and 647 (krypton) nm, are delivered to the system via fiber optics from lasers in adjacent laboratories. A 600 grooves/mm grating blazed at 500 nm is often used (the third grating available to us is 300 grooves/mm blazed at 1 m). The switch between Raman and fluorescence can be made in less than two minutes. REFERENCES Brennan JF, Wang Y, Dasari RR and Feld MS, “Near-Infrared Raman Spectrometer Systems for Human Tissue Studies”, Appl Spectrosc, 51(2), 201-208 (1997b).