Ferrocene and Ferrocene Carboxylic Acid
advertisement

Inorganic-Chemical Practical Course
Spring Semester 2014
Ferrocene and Ferrocene Carboxylic Acid
29.04.2014
Yann Baumgartner
A1
Yann Baumgartner
Exp #4
1 Aim of the experiment
Ds ziu vo däm Experimänt isch d’Synthesä vo Ferrocene (4) und Ferrocene Carbonsüri (5).
Derfür heimer zersch Dicyclopentadien (1) müesse cräcke um Cyclopentadien (2) z’erhaute.
Das heimer när brucht um Ferrocene z’härsteue. Mit däm Ferrocene (4) heimer när
d‘Ferrocene Carbonsüri synthetisiert.
2 Introduction
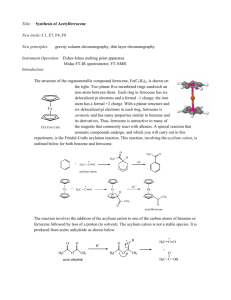
Ferrocene isch in 1951 entdeckt worde und het nach der Strukturufklärig e nöie Pfad ir
Chemie göffnet, nämlech d’Organometall Chemie. Ferrocene ghört zur Gruppe vo
Metallocene und het die typischi
„Sandwich“ Struktur wie ir Figur
1 sichtbar. Angeri derivate hei e
ähnlichi Struktur, was sie aber aui
gmeinsam hei, isch d’Bindigsart
zum
Cyclopentadien
D’Bindig
isch
nid
(=CP).
lokalisiert
sondern es isch ds π-System vom CP-Ring vo mit de läre Orbitale vom Metaukation binded.
Dämentsprächend sie ou aui C-Atome vom Ring chemisch equivalänt. Um d’azauh vo de
bindende C Atome z’beschribä, die sogenannti haplicität, brucht me der griechisch
Buechstabe η (eta). Ferrocene wird nach IUPAC Empfählige Fe(η5-C5H5)2 gschribe. Die
ähnlechi Reaktivität vom CP-Ring mit dere vo Benzol het zum name Ferrocene gfüert, da im
änglische Benzol Benzene heisst. Hüt zu tags wärde Metallocene sehr oft brucht, und sie si
Bestandteil vo viune aktuellä Forschige wie in Krebs Bekämpfig, in Polymer Katalyse, in
kontrollierter Verbrönnig vo Minerau Öu und in asymetrischer Katalyse. D’Ferrocene
Carbonsüri wird hüfig aus Vorstuefe für Ferrocene Derivate brucht, da d’Synthese dervo
eifach isch, und e nöie funktionelli Gruppe igfüert wird.[1-4] Ferrocene het ideali Eigeschafte
für analytischi Electrochemie, wöu d‘Redox Reaktione voustädnig reversibu si und wöus in
wässrigem Milieu stabiu isch. So wird zum Bispiu es Derivat hützutags aus Biosensor in
Mässgräte brucht um der Zuckerghaut vom Bluet z‘bestimme, was für Diabetiker sehr
nützlech isch. Da me Ferrocene sehr eifach eifach cha Verändere öffnet sech e breiti Pallette
vo Awändige wo noni ungersuecht sie worde.[5]
2
Yann Baumgartner
Exp #4
3 Experimental parts
3.1 Preparation of cyclopentadiene
C10H12
C 5 H6
132 g/mol
66.1 g/mol
18.8 g
20 mL
142 mmol
Dicyclopentadiene (1, 20 mL, 19.6 g, 148 mmol) was heated to 170°C and cracked in a retro
Diels-Alder reaction. The obtained cyclopentadiene (2) was collected in cooled round-bottom
flask and stored in the fridge until further use to inhibit the thermal [2+2] cycloaddition. A
1
1
H-NMR spectrum was measured to check the purity.
H-NMR (250 MHz, 295 K, CDCl3, /ppm): 6.63 – 6.57 (m, 2H, 3), 6.52 – 6.46 (m, 2H, 2),
3.01 (tt, 2H, 4JHH = 1.45 Hz, 3JHH = 2.9 Hz, 1),
ac_prakt_.2636
3
Yann Baumgartner
Exp #4
3.2 Synthesis of ferrocene
C5H6
FeCl2 * 4H2O
C10H10Fe
66.1 g/mol
199 g/mol
186 g/mol
1.18 g
1.79 g
1.38 g
17.8 mmol
9.02 mmol
7.42 mmol
2.0 eq.
1.0 eq.
82 %
1.5 mL
This reaction was carried out under inert conditions. FeCl2 . 4H2O (3, 1.79 g, 9.02 mmol, 1.0
eq) was stirred in DMSO (7.0 mL) for two hours for partial dehydration resulting in increased
reactivity. Cyclopentadiene (2, 1.5 mL, 1.18 g, 17.8 mmol, 2.0 eq) was added to a mixture of
pulverised potassium hydroxide (6.70 g, 118 mmol, 6.7 eq.) in 1,2-dimethoxyethane (20 mL)
and stirred until the reaction mixture was red. The previously prepared solution of FeCl2
.
4H2O (3) in DMSO was slowly added to the reaction mixture and stirred for 30 minutes. The
reaction mixture was added to a mixture of hydrochloric acid (6 M, 25 mL) and ice (30 g) and
stirred for 15 minutes. The precipitate was collected by filtration, washed with water (3x, 3.5
mL) and dried to obtain ferrocene (4, 1.38 g, 7.42 mmol, 82 %, Lit[6]: 89 – 98 %) as an orange
solid.
Mp.: 173 °C (Lit[6]: 173 - 174 °C)
1
H-NMR (250 MHz, 295 K, CDCl3, /ppm): 4.16 (s, 10H, 1),
IR ( /cm-1):3095 (w, C-H
arom.),
(st,δoop, C-H) 475 (st, C=C arom).
1409 (w, C=C
arom),
ac_prakt_.2649
1106 (m, δC-H), 1001 (w, δC-H), 815
4
Yann Baumgartner
Exp #4
3.3 Synthesis of ferrocene carboxylic acid
C10H10Fe
C11H10FeO2
186 g/mol
230 g/mol
1.00 g
1.97 g
5.36 mmol
8.53 mmol
1.0 eq.
not determined
This reaction was carried out under inert conditions. Ferrocene (4, 1.00 g, 5.36 mmol, 1.0 eq.)
and t-BuOK (0.060 g, 0.538 mmol, 0.1 eq.) were dissolved in THF (50 mL) and cooled to
78°C.When the mixture reached 78°C, t-BuLi (6.8 mL, 10.8 mmol, 2.0 eq.) was slowly
added. The reaction mixture was stirred for 30 minutes. A stream of CO2 was bubbled through
the reaction mixture while allowing it to heat to room temperature. Water (18.5 mL) was
added and the aqueous layer was collected. The organic layer was extracted with aqueous
sodium hydroxide (10 %, 3x, 18.5 mL). The combined aqueous layers were acidified with
concentrated hydrochloric acid until everything precipitated. The obtained solid was collected
by filtration and intensively washed with water to obtain ferrocene carboxylic acid (5, 1.97 g,
8.53 mmol, 159 %, Lit[4]: 90 %) as an ochre solid.
Mp.: 188 °C (Lit[4]: 190 °C)
1
H-NMR (250 MHz, 295 K, CDCl3, /ppm): 4.85 (t, 2H, 3JHH = 1.9 Hz, 2), 4.46 (t, 2H, 3JHH =
1.9 Hz, 1) 4.25 (s, 5H, 3),
ac_prakt_.2665
IR ( /cm-1):3347 (m, O-H) ,1651 (st, C=O), 1473 (m, C=C arom), 1280 (m, δO-H), 1158 (w, CC)
5
Yann Baumgartner
Exp #4
4 Discussion
4.1 Preparation of cyclopentadiene
The cracking of dicyclopentadiene (1) worked fine and enough cyclopentadiene (2) was
collected for all the lab-mates. It was important not to heat above 170°C as dicyclopentadiene
(2) boils at 176°C and would have been distilled with cyclopentadiene (1). It is aso important
to collect the cyclopentadiene (2) in a cooled flask to avoid the thermal dimerization. The
recorded 1H-NMR spectrum showed that it was pure enough for further use.
4.2 Synthesis of ferrocene
This part of the reaction was conducted under inert conditions. The potassium hydroxide was
pulverised to increase the contact surface as it does not fully dissolve in DME. The iron salt 3
was stirred for two hours in DMSO to increase the reactivity by dehydration. It was very
important to completely dry ferrocene (4) for the next step. The IR spectrum showed that the
product was dry. In this type of ferrocene synthesis the potassium hydroxide deprotonates
cyclopentadiene resulting in a negatively charged aromatic ring which forms π-bonds with the
iron cation.
6
Yann Baumgartner
Exp #4
4.3 Synthesis of ferrocene carboxylic acid
The inert atmosphere was very important for this step as the t-BuLi and t-BuOK would react
with the present water in the air. This is also the reason why the previously prepared ferrocene
(4) had to be completely dry. The yield was not determined because of considerable remains
of solvent (H2O) in the solid. This also explains the wide O-H stretch in the IR spectrum. The
important water peak in the 1H-NMR spectrum is partially due to the same reason, but also to
some present water in the CDCl3 . However after drying it for 1.5 hours with the schlenk line,
I decided to move on with the analytics. For further use however it would have to be dried. In
this synthesis one cyclopentadiene ring of the ferrocene (4) is lithiated from t-BuLi resulting
in a reactive intermediate product 6 who easily reacts with an electrophile, in this case CO2, to
give ferrocene carboxylic acid (5).
5 Conclusion
The synthesis of ferrocene and ferrocene carboxylic acid were successfully achieved. We also
got to learn how to work with dangerous chemicals such as t-BuLi and we had again the
opportunity to practice working under inert conditions. We also learned how little change on
the main structure such as the addition of a -COOH group can completely change the outcome
of 1H- and 13C{1H}-NMR spectra.
6 References
[1]
C. Ornelas, New J. Chem., 2011, 35, 1973 – 1985
[2]
D.C. Bowman, J. Chem. Educ., 2006, 83, 735.
7
Yann Baumgartner
Exp #4
[3]
R. Tong et al., J. Organometallic Chem., 2014 755, 16 - 32
[4]
B. Breit, D. Breuniger, Synthesis, 2005, 16, 2782 – 2785
[5]
S. Matic, M. Labib, P. O. Shipman, H-B. Kraatz, The Roy. Soc. Of Chem., 2011, 40,
7264 - 7265
[6]
Jolly, Inorg. Synth., 1968, vol. 11, 120.
8
Yann Baumgartner
Exp #4
7 Answers to the questions
7.1 The 13C{1H}NMR spectrum of Cp2Fe in CDCl3 is shown below. What does the {1H}
mean? Is the spectrum consistent with the structure of ferrocene?
The {1H} means that the spectrum was proton decoupled. That way the couplings with
protons are not visible and therefore the spectrum is easier to interpret. The spectrum is
consistent with the structure of ferrocene as the bonds between Fe and Cp are not localized. In
fact it is the π-system of Cp that interacts with Fe, meaning that all carbon atoms in the Cp
ring are equivalent. This explains why there is only one peak for the 10 carbon atoms of the
ferrocene.
9
Yann Baumgartner
Exp #4
7.2 Assign the MAJOR peaks in the EI mass spectrum of Cp2Fe.
The peak at 186 m/z belongs to the cation [Cp2Fe]+. Cp rings dissociate easily and therefore
the peak at 121 m/z belongs to the [CpFe]+ ion and the peak at 56 m/z to the [Fe]+ ion. The
peak at 93 m/z belongs to the double charged molecule-ion [Cp2Fe]2+. The observable peak
patterns occur due to the different isotopes of Fe and C and their abundance.
7.3 In what oxidation state is Fe in:
FeII
FeII
FeIII
10
Yann Baumgartner
Exp #4
7.4 Assign the signals in the following 13C{1H}NMR spectra to the structures shown. You
may not be able to assign all signals unambiguously.
11
Yann Baumgartner
Exp #4
8. Spectra
i)
1
ii)
IR spectrum of ferrocene (4)
iii)
1
iv)
IR spectrum of ferrocene carboxylic acid (5)
v)
1
H-NMR spectrum of cyclopentadiene (2)
H-NMR spectrum of ferrocene (4)
H-NMR spectrum of ferrocene carboxylic acid (5)
12
35000
0.11
0.04
1.51 HDO
3.01
6.59
6.51
6.50
7.26 CDCl3
ac_praktI_.2636.1.fid
Yann, CP
30000
15000
10000
25000
5000
20000
2.00
1.99
0
6.75 6.70 6.65 6.60 6.55 6.50 6.45 6.40
f1 (ppm)
15000
30000
20000
10000
10000
5000
2.20
0
3.05
3.00
f1 (ppm)
2.95
2.90
0
12.0 11.5 11.0 10.5 10.0
9.5
9.0
8.5
8.0
7.5
7.0
6.5
2.20
3.10
1.99
2.00
3.15
6.0 5.5
f1 (ppm)
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
-0.5
PerkinElmer Spectrum Version 10.03.08
Wednesday, April 09, 2014 4:38 PM
Student
Wednesday, April 09, 2014 4:38 PM
99
95
90
1714.45cm-1
854.76cm-1
3094.72cm-1
1408.62cm-1
85
787.56cm-1
80
%T
75
70
1000.68cm-1
65
1105.68cm-1
60
487.90cm-1
55
50
814.76cm-1
45
43
4000
3500
3000
2500
2000
1500
1000
474.87cm-
500 450
cm-1
Yann B. Fer.
Yann B. Fer, April 09 2014
The Quality Checks do not report any warnings for the
sample.
7500
0.07
0.00
1.56 HDO
2.62 Acetone
4.16
7.26 CDCl3
ac_praktI_.2649.1.fid
YBV4.1
7000
6500
6000
5500
5000
4500
4000
3500
3000
2500
2000
1500
1000
500
0
-500
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
f1 (ppm)
3.0
2.5
2.0
1.5
1.0
0.5
0.0
PerkinElmer Spectrum Version 10.03.08
Monday, April 14, 2014 2:52 PM
Student
Monday, April 14, 2014 2:52 PM
98
95
90
85
80
75
1029.38cm-1
913.96cm-1
%T
70
933.99cm-1
65
1473.14cm-1
60
1157.67cm-1
3347.21cm-1
825.06cm-1
782.09cm-1
1280.88cm-1
55
739.05cm-1
50
597.65cm-1
1650.97cm-1
45
562.20cm-1
478.73cm507.92cm-1
40
36
4000
3500
3000
2500
2000
1500
1000
500 450
cm-1
Yann B.
Exp. #4.2 By Yann B. Date Monday, April 14 2014
The Quality Checks do not report any warnings for the
sample.
1700
-0.00
400
1800
1.25 t-BuOK
1.71 O-H
4.86
4.85
4.84
4.47
4.46
4.45
4.25
7.26 CDCl3CDCl3
ac_praktI_.2665.1.fid
Yann B, E#4 Endprodukt
1600
300
1500
200
1400
100
1300
4.8
4.7
4.6
4.5
f1 (ppm)
1200
5.06
1.99
2.00
0
4.4
1100
4.3
1000
900
800
700
600
500
400
300
200
100
9.0
8.5
8.0
7.5
7.0
6.5
6.0
5.5
5.0
4.5
-100
5.06
1.99
2.00
0
4.0
f1 (ppm)
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
-0.5