NOMENCLATURE RULES

a.
NOMENCLATURE RULES
ACID RULES
-
An acid is an ionic compound with H +1 acting as the metal at the beginning. You can have more than one H +1 at the beginning, as in H
2
(SO
4
) and H
3
(PO
4
).
-
You always have one or more H +1 , depending on the negative ion present
HERE ARE THE TWO RULES WHEN NAMING AN ACID:
1.
Name the negative ion that has reacted with hydrogen
2.
Change the ending of the ion according to the rules.
If the ending on the reacted atom with oxygen is -ate , simply drop the -ate ending and add –ic.
Let’s look at H
2
(SO
4
):
(SO
4
-2 ) is the sulf ate ion. drop the -ate , and add -ic . H2SO4 would be sulfur ic acid. b.
c.
If the ending on the reacted atom with oxygen is -ite , simply drop the -ite ending and add –ous . Let’s look at H(NO
2
):
(NO
2
) -1 is the nitr ite ion. Drop the -ite , and add -ous . HNO2 would be nitr ous acid.
When naming an acid that has a reacted negative atom without oxygen, the -ide goes to -hydro-ic . Let’s look at HCl:
Cl-1 is chlor ide . Drop the ide ,, and add –hydro –ic . HCl would be hydro chlor ic acid
Remember: hydro in the acid name means NO OXYGEN – that is why it came from –ide!
In summary, remember: ate to ic, ite to ous, and ide to hydro-ic!
COVALENT RULES
-
A covalent compound has two non-metals
-
All covalent compounds, whether polar or non-polar, are named the same way:
-
Simply name both elements, placing prefixes before them that correspond to the number of atoms of that particular element. Then, drop the ending of the last element and add -ide.
-
THIS CAN BE SUMMARIZED INTO ONE RULE:
1. Prefix – element prefix – element – ide ie: N2O3 would be di nitrogen tri ox ide.
- if there is only one element, do not use mono prefix unless it is the last element. ie: CO would be carbon mon oxide, NOT mono carbon mon oxide.
IONIC RULES!
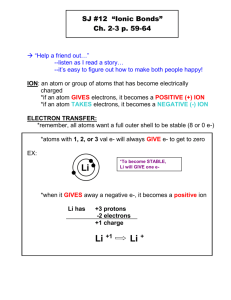
-
An ionic compound has a metal and a non-metal . The first will always be a metal, the second a non-metal. Ionic compounds always give and take electrons. There is no sharing. When they do this, they form a positive ion and a negative ion, and then attract to each other to form a ionic bond.
An ion is just a charged atom, and an ionic compound has a positive ion attracted to a negative ion.
-
The charge is understood – it is never written. For example, when you see NaCl, scientists expect for you to know that this means Na give away. It forms Al +3
+1 and Cl -1 . Sometimes, it takes more than one atom to make another atom happy. For example, you can have AlBr
3
. Why? Al has three valence electrons to
when it gives away those electrons. Br has 7 valence electrons. It needs just one more electron, and when it gains that electron, it becomes Br -1 . So, it would take three Br atoms in order to take all of aluminum’s valence electrons!
-
THERE IS ONE RULE WHEN NAMING IONIC COMPOUNDS: Name both ions!
-
When you have an ionic compound that only two elements, and the non-metal does not contain oxygen, that is called a binary ionic compound. An example would be LiBr. To name this compound, you name both ions. The name of the metal ion is just the name of the metal! The name of the negative ion varies. If the negative ion is just a reacted non-metal without oxygen, it has ide at the end. So this compound would be called lithium bromide, the ide ending meaning bromine has reacted, but there is no oxygen!
Summary: ide at the end of an ionic compound means reacted, with no oxygen!
- Some ionic compounds contain a polyatomic ion , which is a group of non-metals acting like one big non-metal that can take electrons. For example, in the compound, Mg(SO
4
), the magnesium is giving away two electrons not to one non-metal, but to a group of non-metals. The polyatomic ion present in this compound is called sulfate, which is (SO
4
) -2 . How do you name them? a. There is a four on the periodic table – P, S, As, Se, and Te. When these elements are with oxygen, they normally have 4 oxygens attached. We use the name –ate at the end to indicate that the atom is bonded to the normal number of oxygens. If the element is NOT in the four, it normally has 3 oxygens when we use the ending –ate . SO, -ATE AT THE END MEANS WITH OXYGEN!
Example: Chlor ate is (ClO
3
) -1 , because chlorine is not in the four, and therefore it normally has 3 oxygens, and we end it with ate. Phosphate is (PO
4
) -3 , because phosphorous is in the four. a. If there is one less oxygen than the normal, the name ends in –ite. For example, (SO
3
) -2 is called sulfite, because it has one less oxygen than normal. If there is two less oxygens than normal, we use the name hypo-ite.
For example, (SO
2
) -2 would be called hyposulfite. If there is one more oxygen than normal, we use the name less oxygen, and one more sulfur, we use the name thio. For example, (S
2
O
3
) thiosulfate. If there is a H +1 per. For example, (SO
5
) -2 would be called persulfate. If there is one
-2
attached, we use the name bi . For example, (HSO
4
) would be called
-2 would be called bisulfate. You can change the names for any polyatomic ion using these name changes! b. To figure out the charge on the polyatomic, you sing the song Mr. Jauss taught = -1, -2, -3, -2, -1 = this tells you the charge on the polyatomic ion, no matter how many oxygens it has! So sulfate, sulfite, hyposulfite, persulfate all have a –2 charge!