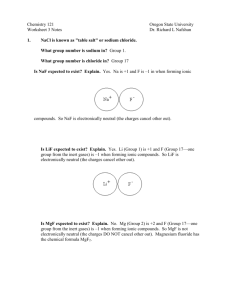
Intro Practice Quiz - Mr. Lesiuk
advertisement

Practice Quiz 1. The element SODIUM (Na) has the atomic number 11 What does that mean? 2. If that atom is electrically neutral; how many electrons will it possess, and how many of those will be found in its outermost shell. ** It is found in Group Roman Numeral (I) = 1 on the periodic table. 3. What does it need to do to satisfy the "Octet Rule"? 4. The element CHLORINE (Cl) has the atomic number 17. What does that mean? 5. If that atom is electrically neutral; how many electrons will it possess, and how many of those will be found in its outermost shell, when it is electrically neutral? *** It is found in Group Roman Numeral (VII) = 7 6. What does it need to do to satisfy the "Octet Rule"? 7. After the metal sodium and the non-metal chlorine satisfy the octet rule, what type of charge will each atom have? A) Sodium ? B) Chlorine? 8. What type of bond will form between these two ions? NOW FOR WATER !!!