Periodic Table of the Elements
advertisement
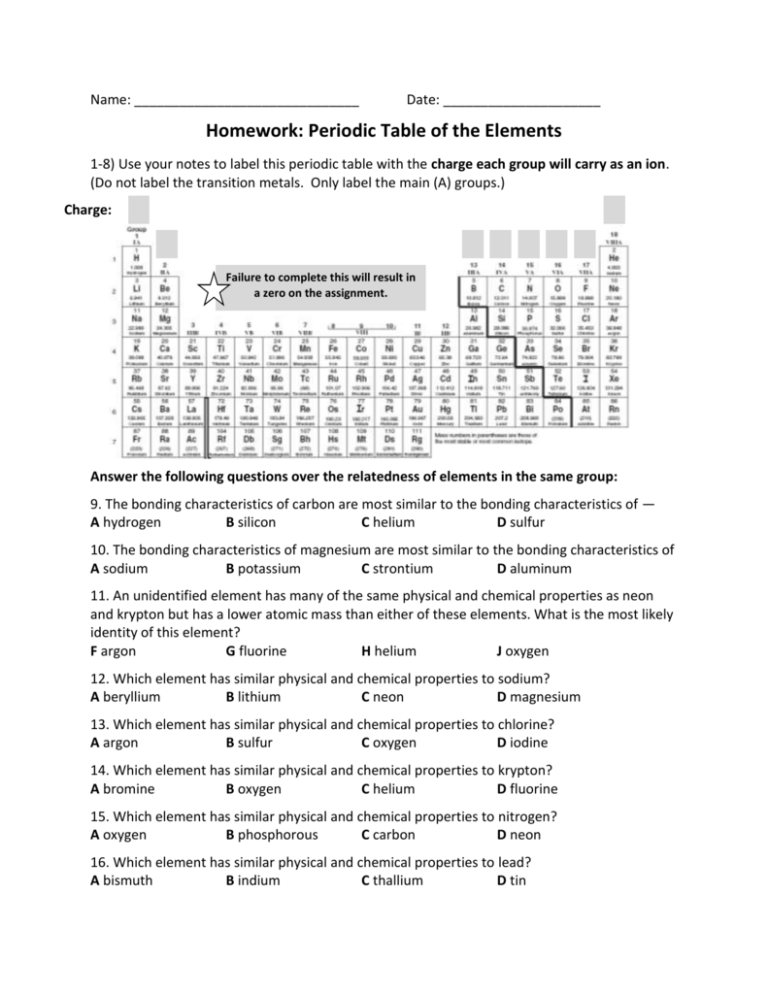
Name: ______________________________ Date: _____________________ Homework: Periodic Table of the Elements 1-8) Use your notes to label this periodic table with the charge each group will carry as an ion. (Do not label the transition metals. Only label the main (A) groups.) Charge: Failure to complete this will result in a zero on the assignment. Answer the following questions over the relatedness of elements in the same group: 9. The bonding characteristics of carbon are most similar to the bonding characteristics of — A hydrogen B silicon C helium D sulfur 10. The bonding characteristics of magnesium are most similar to the bonding characteristics of A sodium B potassium C strontium D aluminum 11. An unidentified element has many of the same physical and chemical properties as neon and krypton but has a lower atomic mass than either of these elements. What is the most likely identity of this element? F argon G fluorine H helium J oxygen 12. Which element has similar physical and chemical properties to sodium? A beryllium B lithium C neon D magnesium 13. Which element has similar physical and chemical properties to chlorine? A argon B sulfur C oxygen D iodine 14. Which element has similar physical and chemical properties to krypton? A bromine B oxygen C helium D fluorine 15. Which element has similar physical and chemical properties to nitrogen? A oxygen B phosphorous C carbon D neon 16. Which element has similar physical and chemical properties to lead? A bismuth B indium C thallium D tin 17. Which of the following groups contains members with similar chemical reactivity? A K, Ca, Sc B V, Cr, Mn C Cu, Ag, Au D N, O, F 18. Which of the following groups does not contains members with similar chemical reactivity? A Ni, Pd, Pt B C, N, O C Li, Na, K D F, Cl, Br 19-32)Using your periodic table and your notes from today, decide if the following elements are part of a group that will donate 1 electron, donate 2 electrons, gain 1 electron, gain 2 electrons, or does not gain or lose. Mg ____________________________ F ____________________________ S ____________________________ Ne ___________________________ K ____________________________ O ____________________________ Ar ____________________________ Be ___________________________ Li ____________________________ Se ____________________________ Cl ____________________________ Ca ___________________________ Na ____________________________ I ___________________________ 33-56) Use your periodic table to predict the charges of the following ions: Be _____ I _____ Ar _____ Na _____ Cl _____ Ca _____ O _____ K _____ F _____ S _____ P _____ Mg _____ Se _____ Br _____ He _____ Li _____ N _____ Sr _____ Xe _____ Ba _____ Al _____ Kr _____ H _____ Rb _____ 57-68) What are the charges of the following transition metals? Iron (II) ________ Tin (IV) ________ Cobalt (II) ________ Palladium (I) ________ Lead (II) ________ Copper (I) ________ Vanadium (IV) ________ Platinum (II) ________ Manganese (III) ________ Copper (II) ________ Iron (III) ________ Tin (II) ________ 69) Why do transition elements always have a positive charge? __________________________ ______________________________________________________________________________ 70)Which group on the periodic table contains inert elements? __________________________