tpj12966-sup-0009-Legends
advertisement

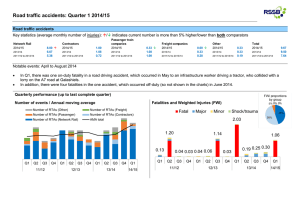
1 SUPPORTING FIGURE LENGENDS 2 Figure S1. Schematic of the predicted domains of CMPG proteins and phylogenetic analysis of 3 CMPG amino acid sequences from plants. Nucleotide sequence alignment of CMPG1-V and its 4 orthologs in common wheat. 5 (a) Schematic representation of CMPG1-V and other U-Box/ARM proteins from plants. 6 CMPG1-V (H.villosa), AK375870 (Hordeum vulgare), Os02g0738200 (Oryza sativa), 7 NM_001154388 (Zea mays), XM_003573078 (B. distachyon), PcCMPG1 (Petroselinum crispum), 8 SlCMPG1 (Solanum lycopersicum), NtCMPG1 (Nicotiana tabacum), AtCMPG2, AtPUB17, AtPU22 9 and AtPUB12 (Arabidopsis thaliana), StPHOR (Solanum tuberosum) were used for analysis. The 10 orange columns at N-terminus represent the U-Box domain, and the percentages in orange columns 11 represent the similarities of U-Box domain. Purple columns represent the ARM repeats, and the 12 number of ARM repeats is represented by the numbers in the purple columns, the percentages in the 13 second purple column represent the similarities of ARM repeat domain. The numbers show the start 14 and stop amino acids of each domain, and the most right percentages represent the similarities of the 15 full length of different U-Box/ARM proteins with CMPG1-V. 16 (b) Phylogenetic analysis of CMPG amino acids sequences from plants. The scale bar of 0.1 indicates 17 10% sequence divergence. 1000 bootstrap repetitions were made. 18 (c) Nucleotide sequence alignment of CMPG1-V and its orthologs from common wheat Chancellor, 19 Funo, Baimian 3 (BM), Kavkaz and Ulka/8cc (UL). The red lines indicate positions of primers 20 CMPG-NP-F/CMPG-NP-R. 21 Figure S2. Sequence alignment of the specific amplicons amplified using primers 22 CMPG-NP-F/CMPG-NP-R. 23 From up to down: CMPG sequences amplified from plasmid pBI-220-CMPG1-V, H.villosa and 24 T6AS·6VL. 25 Figure S3. Schematic of the pedigree of the CMPG1-V transgenic plants from T0 to T4 26 generation. 27 T0-26, T0-46 and their derived lines were identified as positive transgenic plants while T0-33 and its 28 derived plants were negative control. 29 Figure S4. PCR identification of CMPG1-V transgenic lines T2-26 and T2-46. 30 NAU02Y393, Yangmai158 and T2-N (negative transgenic plant) represent negative control. Plasmid 31 (pBI-220-CMPG1-V), T1-26-3 and T1-46-25 represent positive control. The arrow indicates that the 32 target band of amplification (206bp). 33 Representative PCR results of plants derived from (a) T1-26-3 (T2-26-1~ T2-26-6) and plants from (b) 34 T1-46-25 (T2-46-1~ T2-46-6) were shown. 35 Figure S5. Determination of H2O2 content and activities of antioxidase enzymes in leaves of 36 Yangmai158, T4-N, T4-26, T4-46, T6AS·6VL, NAU9918 and 91C43 (H.villosa) after inoculation of 37 Bgt. 38 (a) The content of H2O2, (b) enzyme activity of guaiacol peroxidase (POD) and (c) ascorbate 39 peroxidase (APX) in leaves of Yangmai158, T4-N, T4-26, T4-46, T6AS·6VL, NAU9918 and 91C43 40 (H.villosa) were assayed. Values are means ±SE of three independent experiments with at least three 41 replicates for each. Different letters represent statistically significant differences (P<0.05, one-way 42 ANOVA). 43 Figure S6. Expression patterns of CMPGs in different materials in response to Bgt infection. 44 RT-qPCR showed the relative expression of CMPG genes in 91C43 (H.villosa), NAU9918, T6AS·6VL 45 and Yangmai158 leaves. Tubulin gene was used as a reference gene. The RT-qPCR values were 46 normalized to tubulin and then presented as fold-changes relative to 91C43, NAU9918, T6AS·6VL and 47 Yangmai158 without treatment respectively. Similar results were obtained from three independent 48 experiments, and standard deviation (n = 3) is represented by error bars. The letters represent 49 statistically significant differences (P<0.01, one-way ANOVA) of 91C43, NAU9918, T6AS·6VL and 50 Yangmai158 respectively. 51 Figure S7. Expression patterns of CMPGs in T4 transgenic plants in response to Bgt treatment. 52 RT-qPCR showed the relative expression of CMPG genes in Yangmai158, negative transgenic plants 53 (T4-N) and positive transgenic plants T 4-46 and T4-26 leaves. The RT-qPCR values were normalized to 54 tubulin and then presented as fold-changes relative to Yangmai158 without treatment. Values represent 55 the mean and standard deviations obtained from three replicates of each sample. Different letters 56 represent statistically significant differences (P<0.01, one-way ANOVA).