Ibrance is the only non-hormonal targeted therapy approved for use
advertisement

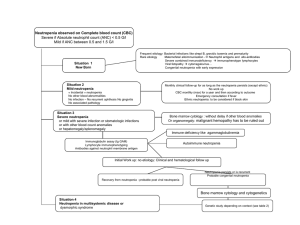
New Drug Introduction: Ibrance / palbociclib Pharmacology Manufacturer Approval Date Indications Contraindications Warnings And Precautions Pregnancy/Lactation Pharmacokinetics Drug Interactions – Object Drugs Palbociclib is a reversible inhibitor of cyclin-dependent kinase (CDK) 4 and 6. CDK 4/6 are downstream of signaling pathways that regulate cellular proliferation. Palbociclib reduces proliferation of estrogen receptor (ER) positive breast cancer cells by blocking progression of the cell from G1 into S phase of the cell cycle. The combination of palbociclib and letrozole increased the inhibition of downstream signaling and tumor growth in ER positive breast cancer xenograft models compared to either drug alone. Pfizer Labs February 2015 Palbocilib is indicated for use as initial endocrine based therapy (in combination with letrozole) for the treatment of estrogen receptor (ER)positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer in postmenopausal women. none Bone marrow suppression - neutropenia was commonly observed in clinical studies, including grades 3 and 4 neutropenia Monitor blood counts; treatment interruption, delay, or dose reduction is recommended for patients developing grade 3 or 4 neutropenia. Infections (including grades 3 and 4) were reported more frequently in patients receiving palbociclib and letrozole compared with those receiving only letrozole. Monitor for signs/symptoms of infection and manage appropriately. Thromboembolic events - pulmonary embolism was observed more frequently in patients receiving palbociclib and letrozole compared with those receiving letrozole alone. Monitor for signs/symptoms of pulmonary embolism and manage appropriately. Gastrointestinal toxicity - nausea, vomiting, diarrhea, and stomatitis (generally grade 1 or 2) were reported from clinical studies. Embryo-Fetal toxicity – can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. Category: Not currently determined (likely X) - Adverse events were observed in animal reproduction studies Lactation: Excretion in breast milk unknown/not recommended A – Bioavialibility: 46%. Cmax: 6-12 hours D – 85% protein bound with mean Vd of 2583 L M – Undergoes extensive hepatic metabolism predominately via CYP3A4 and SULT2A1; major metabolite glucuronide conjugate of palbociclib E –Feces (74% as metabolites); Urine (17.5% as metabolites); Palbociclib has a mean plasma half-life of 29 +/- 5 hrs. levels of CYP3A4 substrates; dose adjustment may be required for those agents with narrow therapeutic indices midazolam cyclosporine fentanyl everolimus alfentanil pimozide ergotamine sirolimus tacrolimus Drug Interactions – Precipitant drugs Adverse Effects Monitoring Efficacy Monitoring Toxicity Dosing - Initial Dosing - Usual Dosing - Max Renal Adjustment Hepatic Adjustment Strong CYP3A4 inhibitors concentration of Ibrance ketoconazole itraconazole clarithromycin ritonavir nefazodone grapefruit Strong CYP3A4 inducers: ↓ concentration of Ibrance phenytoin rifampin carbamazepine St. John’s Wort Nausea (25%) [13%] Neutropenia (75%) [5%] Diarrhea (21%) [10%] Leukopenia (43%) [3%] Vomiting (15%) [4%] Anemia (35%) [7%] Stomatitis (25%) [7%] Thrombocytopenia (17%) [1%] Fatigue (41%) [23%] Decreased appetite (16%) [7%] Asthenia (13%) [4%] Peripheral neuropathy (13%) [5%] URTI (31%) [18%] Alopecia (22%) [3%] Epistaxis (11%) [1%] Monitor for signs of clinical improvement/lack of disease progression CBC at baseline and start of each cycle; day 14 of first two cycles. 125 mg once daily for 21 days followed by 7 days off treatment to complete 28 day cycle. Letrozole 2.5 mg/day should be taken continuously during 28 day cycle. 125 mg/day unless neutropenia or other myelosupression develops. If ANC <1000/mm2 + fever > 38.5 oC or infection withhold until ANC > 1000 mm2. Resume at 100 mg/day. Dose can be further reduced to 75 mg/day on second occurrence, however if third occurrence discontinue use. 125 mg once daily No dosage adjustment required for patients with mild to moderate renal impairment (30 ml/min < CrCl < 60 ml/min). Ibrance has not been studied in patients with severe renal impairment. No dosage adjustment required for mild hepatic impairment. Ibrance has not been studied in patients with moderate or severe hepatic impairment. Summary Ibrance in combination with letrozole is indicated for the treatment of postmenopausal women with estrogen positive, human epidermal growth factor negative metastatic breast cancer. Ibrance is the only non-hormonal targeted therapy approved for use in women with ER-positive, HER-2 negative advanced breast cancer. First CDK inhibitor approved for use in oncology. Significant side effects include neutropenia and leukopenia. Dose reduction is suggested as first step in management. References: 1. http://www.Ibrance.com 2. Ibrance full prescribing infromation. Pfizer. Feb. 2015. 3. Palbociclib. Drug information. Lexicomp Drug Information. Accessed through UpToDate. Accessed on February 15, 2015.