Electronic Supplementary Material
advertisement

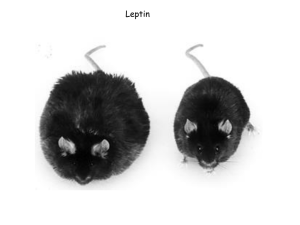
Electronic Supplementary Material Birds were maintained in individual cages in an indoor aviary, receiving water and food (a commercial mix; Benelux NV, Belgium) ad libitum throughout the experiment. Birds were implanted with a 10-mm subcutaneous silastic tube (1.47-mm i.d., 1.96-mm o.d.; Silastic laboratory tubing, Dow Corning) filled with testosterone (T, n = 12) (SIGMA, T1500) or empty (C, n =12). Implants were sealed at both ends with 1 mm silicon glue (Nusil ref. MED1000, Nusil Technology, Canada). Therefore, the actual portion filled approximated 8 mm. Same-sized testosterone implants have been successfully used in other studies on zebra finches (e.g. McGraw et al. 2005). Testosterone-implanted males showed higher levels of testosterone than controls one week before the start of the experiment (F 1,22 = 88.9, P < 0.001; mean and range; T: 24.7, 10.5 - 35 ng/ml; C: 1.15, 0.16 - 7.66 ng/ml; see also Alonso-Alvarez et al. 2007). Testosterone implants were still filled at the end of the experiment. Each bird received daily injections of 15 µg of murine leptin (Prospec-Tany Technogene LTD, Israel, ref. CYT-351) diluted in 100 µl PBS. Leptin dose was calculated from the recent Lohmus et al. (2004)’s study. They found that daily administrations of 1 g of murine leptin per gram of body mass increased cell-mediated immune response, as assessed in this study (PHA injections), in asian blue quails (Coturnix chinensis). Murine and avian leptins have a 95% homology (Doyon et al. 2001). Birds were injected subcutaneously in the wing web with 50 μg of PHA-P (SIGMA, L8754) diluted in 20 μl of PBS, following Smits et al. (1999)’s protocol. The thickness of the wing web was measured at the injection site just prior to and 24 h after the challenge (injections: day 35 and 40, measurements: day 36 and 41). The measurements made in triplicate showed a high repeatability (r = 0.85, p < 0.001; Lessells & Boag 1987), mean values being used for analyses. The wing used to inject PHA was alternated at each sampling bout to avoid potential damages on the skin. Measurements were performed blindly with respect to experimental groups. There was no initial difference in body mass between T- and C-males before leptin administration (p > 0.15). Moreover, there was no initial difference in body mass between T-males injected with leptin and T-males injected with PBS (p = 0.97). Similarly, there was no initial difference in cell mediated immune response between birds that subsequently received leptin or PBS injections (F1,22 = 0.01, p = 0.93). 1 c lept 1.6 c pbs t lept Immune response (mm) 1.4 t pbs 1.2 1 0.8 0.6 0.4 0.2 0 35 40 time Figure 1. Immune response to phytohaemagglutinin skin injections before and after the five day treatment with intraperitoneal leptin injections (means ± SE). PHA-induced immune response (figure 1 above) was higher in the present experiment than in the previous one (figure 1 in Alonso-Alvarez et al. 2007), which illustrates the sensitisation effect of previous exposure (i.e. Johnsen et al. 2000; Alonso-Alvarez & Tella 2001; see also Granbom et al. 2005). Our hypothesis is mainly supported by studies in humans and rats. However, recent findings suggest that leptin should have similar properties in avian species. Thus, circulating leptin levels has been positively correlated to both liver and abdominal fat in hens (Arslan et al. 2004). Both hepatic leptin expression and plasma leptin levels were significantly higher in lines selected for high abdominal fat (Dridi et al. 2005). Meanwhile, the permissive role of leptin in puberty has been recently demonstrated in hens (Paczoska-Eliasiewicz et al. 2006), and recently, Kochan et al. (2006) described leptin synthesis in adipose tissue and liver of a small passerine. Alonso-Alvarez, C. & Tella, J. L. 2001 Effects of experimental food restriction and body-mass changes on avian T-cell mediated immune response. Can. J. Zool. 79, 101-105. Alonso-Alvarez, C., Bertrand, S., Faivre, B., Chastel, O. & Sorci, G. 2007 Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. Lond. B 274, 819-825. Arslan, M., Ozcan, M., Matur, E., Cotelioglu, U. & Ergul, E. 2004 The effects of probiotic on leptin level, body, liver and abdominal fat weights during the rapid growth phase of broilers. Indian Vet J 81, 416-420. Dridi, S., Buyse, J., Decuypere, E. & Taouis, M. 2005 Potential role of leptin in increase of fatty acid synthase gene expression in chicken liver. Domest. Anim. Endocrin. 29, 646-660. Doyon, C., Drouin, G., Trudeau, V. L. & Moon T. W. 2001 Molecular evolution of leptin. Gen. Comp. Endocrinol. 124, 188-198. Granbom, M., Raberg, L. & Smith, H. G. 2005 The spatial and temporal repeatability of PHAresponses. Behav. Ecol. 16, 497-498. 2 Johnsen, A. Andersen, V., Sunding C. & Lifjeld J. T. 2000 Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature 406, 296-299. Kochan, Z., Karbowska, J. & Meissner, W. 2006 Leptin is synthesized in the liver and adipose tissue of the dunlin (Calidris alpina). Gen. Comp. Endocrinol. 148, 336-339. Lessells, C. M. & Boag, P. T. 1987 Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121. Lohmus, M., Olin, M., Sundström, L. F., Troedsson, M. H. T., Molitor, T. W. & El Halawani, M. 2004 Leptin increases T-cell immune response in birds. Gen. Comp. Endocrinol. 139, 245-250. McGraw, K. J., Correa, S. M. & Adkins-Regan, E. 2005 Testosterone upregulates lipoprotein status to control sexual attractiveness in a colourful songbird. Behav. Ecol. Sociobiol. 60, 117-122. Paczoska-Eliasiewicz, H.E., Proszkowiec-Weglarz, M., Proudman, J., Jacek, T., Mika, M., Sechman, A., Rzasa, J. & Gertler, A. 2006 Exogenous leptin advances puberty in domestic hen. Dom. Anim. Endocrinol. 31, 211-226. Smits, J. E., Bortolotti, G. R. & Tella, J. L. 1999 Simplifying the phytohemagglutinin skintesting technique in studies of avian immunocompetence. Funct. Ecol. 13, 567–572. 3