Name ___Per_____Date_________________ 7R CHEMISTRY 1
advertisement

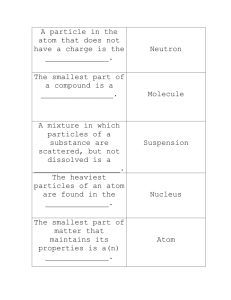
Name _____________________________________________________________Per_____Date_________________ 7R CHEMISTRY 1 REVIEW A. Complete the definitions below about atoms using the words below: NEUTRAL MASS ONE NUCLEUS ELECTRON CLOUD MATTER 1. Matter is anything that has NEGATIVE VOLUME POSITIVE ELEMENT and _______________. 2. An Atom is the smallest unit of . 3. Protons are the sub-atomic particles that have a _______________ charge and are situated in the nucleus of the atom. 4. Neutrons are the sub-atomic particles that are ______________ and are situated in the nucleus of the atom. 5. Electrons have a _______________ charge and are situated outside of the nucleus. 6. An Element is a pure substance that is made of only ________ type of atom (e.g. gold, oxygen). 7. A Compound is a substance that is made of more than one _____________. 8. Protons and neutrons are located in the _______________ of an atom while electrons can be found in the _______________. B. CLASSES OF MATTER 1. Fill in the table below about parts of the atom. SUBATOMIC PARTICLE LOCATION IN THE ATOM CHARGE A. B. C. 2. Label the parts of the atom below C. Identify the class of matter being described: Elements, Compounds or Mixtures _____________ 1. Made up of more than one type of molecule. _____________ 2. Can be separated by physical means. _____________ 3. Made up of only one type of atom. _____________ 4. Made up of more than one type of atom. _____________ 5. Made up of only one type of molecule. D. Multiple Choice 1. When two or more substances are mixed together and are chemically combined, the resulting combination is called a (an) A) element. B) mixture. C) compound. D) phase change. 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. D) mixture. 4. In general, when elements combine chemically, A) they retain all their original properties. C) new substances with new and different properties are formed. B) a mixture results. D) solutions are formed. 5. A mixture of iron filings and sulfur can be easily separated by A) placing the mixture in water. B) using a magnet. C) performing a chemical reaction. 6. When salt is dissolved in water, a(n) A) chemical reaction occurs. B) element forms C) mixture forms. D) heating the mixture. D) compound is formed. 7. The substances in a mixture can be separated by physical means because A) no chemical change occurs when the substances are combined. B) the physical and chemical properties of the substances change. C) none of the properties of the substances change. D) the chemical, but not the physical, properties of the substances change E. 1. Explain the difference between an element and a compound.__________________________________________________ ____________________________________________________________________________________________________ 2. Identify each substance as an element or compound: a. CO2 _____________ b. Na___________ c. CH 4_______________ d. H ______________ 3. Explain the difference between a compound and a mixture. ___________________________________________________ _____________________________________________________________________________________________________ 4. Identify each substance as a compound or mixture: a. Water______________ c. air_____________ e. tea _________________ b. salt water___________ d. carbon dioxide___________ f. salt_________________ F. Identify if each statement represents a chemical or physical property. Write C for chemical property and P for physical property in the space provided. _____1. Water boils at 100 degrees Celsius. _____5. Yeast acts on sugar to form carbon dioxide and ethanol. _____2. Diamonds are capable of cutting glass. _____6. Wood is flammable. _____3. Sugar is capable of dissolving in water. _____7. Ammonia is a gas at room temperature. _____4. Vinegar will react with baking soda. _____8. Bromine has a red color. G. Identify if each statement represents a chemical or physical change. Write C for chemical change and P for physical change in the space provided. _____9. Salt is dissolved in water. _____11. Gasoline burns in the presence of oxygen. _____10. Iron rusts in a damp environment. _____12. Hydrogen peroxide decomposes to water and oxygen. H. Draw the particles of the 3 states of matter in the boxes on the left below. SOLIDS Particles packed tightly together Particles vibrate Particles not held as strongly together as in solids Particles are moving but much more slowly than in gases LIQUIDS GASES Particles are far apart Particles move quickly I.. Write definitions for each of the following terms: 1. Melting – _________________________________________________________________________________________ 2. Freezing – __________________________________________________________________________________________ 3. Evaporation – ________________________________________________________________________________________ 4. Condensation – _______________________________________________________________________________________ 5. Sublimation – ________________________________________________________________________________________ J. The diagram below shows a model in an open container. The model represents the arrangement of particles of matter in a solid phase. a) Draw a diagram showing the arrangement of these particles in a liquid phase. Describe the positioning and motion of the molecules. _________________________________________________________ __________________________________________________________ b) Draw a diagram showing the arrangement of these particles in a gas phase. Describe the positioning and motion of the molecules. _________________________________________________________ __________________________________________________________