Worksheet Chapter 12&13
advertisement

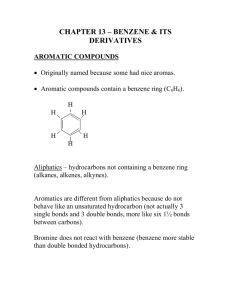
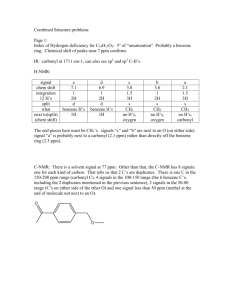
Chemistry 241 Worksheet for Chapters 12 and 13 Page 1: Index of Hydrogen deficiency for C11H12O2: 6° of “unsaturation” Probably a benzene ring. Chemical shift of peaks near 7 ppm confirms this. IR: carbonyl at 1711 cm-1, can also see sp2 and sp3 C-H’s. H-NMR: signal chem shift integration 12 H’s split what a 7.9 1 1H d? not aromatic H max of 5 benz H’s. CH b 7.4 5 5H m benzene H’s c 6.3 1 1H d CH d 4.2 2 2H quartet CH2 e 1.4 3 3H t CH3 next to(split) (chem shift) 1H lots of H’s 1H 3 H’s, (CH3) oxygen 2 H’s, (CH2) So, on one end of our molecule is a benzene ring, on the other is –OCH2CH3. In the middle is a carbonyl (from IR). Still missing are two CH’s and a ring or double bond. Two missing carbons cannot make a ring, so it must be a double bond. You cannot easily tell from the spectra whether it is cis or trans or terminal. That information is in the fingerprint, which we haven’t studied enough. Looking at p541, it looks like it is most likely trans, but this is not definite. Putting the pieces together: O O Page 2: IHD for C7H6O2 is five bonds or rings. Probably a benzene ring. 4 H’s at ~7 ppm confirms this. IR shows broad –OH, maybe even a COOH, but hard to tell. It also looks like a carbonyl at 1680 and an aldehyde H double band at ~ 2800. NMR: signal chem shift integration 6 H’s split what a 11.1 1 1H s aldehyde H b 9.8 1 5H s Ar-OH c 7.4 4 1H m 4 CH’s next to(split) (chem shift) 0 H’s 0 H’s lots of H’s So, end pieces are the aldehyde and the alcohol. The middle piece is the aromatic ring. Whether the ring is 1,2 or 1,3 or 1,4 substituted is present in the fingerprint region, which we have not studied much. However, we can tell from the splitting pattern that the benzene ring is definitely not 1,4 substituted because that would mean two aromatic doublets, which we do not have. So it is 1,3 or 1,2 substituted. I would take either answer. OH OH HO O or OH O Page 3: Index of H deficiency for C10H12O2: 5 pi bonds or rings. Could be benzene ring. Aromatic H’s on NMR at 7.4 and 8.0 seem to confirm this. IR: the usual suspects: sp2 and sp3 C-H’s and a carbonyl at 1720. H-NMR: The signal at 0ppm is always TMS: not due to compound signal chem shift integration 12 H’s split what next to(split) (chem shift) a 8.0 2.4 2H m aromatic H H’s. 2 CH’s lots of H’s b 7.4 3.5 3H m benzene H’s 3 CH’s lots of H’s c 5.3 1.2 1H m CH d 1.4 7.8 6H d 2 CH3’s lots of H’s 1H So we have five aromatic H’s on our benzene ring, which means we have a benzene ring at one end of our molecule. On the other end, my two methyls (signal d) CH must be next to my one , making an isopropyl end. In the middle is my carbonyl (remaining degree of unsaturation) and oxygen. The only thing left to settle is which is next to the CH. Since the chemical shift of the CH is 3.5 ppm, that puts it next to the O, and not the carbonyl. Final structure: O O Page 4: O O Page 5: Index of H deficiency for C9H11NO2: (21-11)/2 = 5 degrees “unsaturation” again. probably a benzene ring, which can be confirmed by the signals near 7 ppm. IR: at 1708 cm-1 is a carbonyl (C=O) again. Also visible is the two peaks of an –NH2 near 3300-3500 cm-1. sp2 and sp3 C-H’s show again H-NMR: signal ch. shift int. split what next to g 7.4 1H d benz. H 1H f 7.3 1H s benz. H 0H e 7.2 1H t benz. H 2H d 6.8 1H d benz. H 1H c 4.4 2H q CH2 3H’s CH3 &O b 3.8 2H s (broad) NH2 a 1.4 3H t CH3 2H’s CH2 One can see that the CH2 and CH3 must be together to make an ethyl. this is one of our end pieces. the other end piece is the NH2. In the middle go the carbonyl, benzene ring, and the O of the ether or ester. From the splitting pattern on the benzene ring (1 singlet), this ring must be 1,3 substituted (meta) from the chemical shift of the CH2, it must be next to the O. given our knowledge, we can come up with two possibilities. with more experience, we might be able to differentiate between the amide and the amine, but that isn’t necessary right now. The IR carbonyl absorption is a little closer to an ester than an amide. O O H2N O H2N or O