Combined Structure problems
advertisement

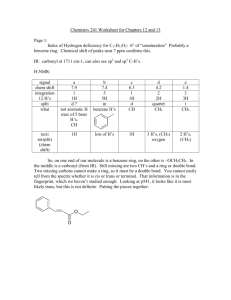
Combined Structure problems Page 1: Index of Hydrogen deficiency for C10H12O2: 5° of “unsaturation” Probably a benzene ring. Chemical shift of peaks near 7 ppm confirms IR: carbonyl at 1711 cm-1, can also see sp2 and sp3 C-H’s. H-NMR: signal chem shift integration 12 H’s split what next to(split) (chem shift) e 7.1 1 2H d benzene H’s 1H d 6.9 1 2H d benzene H’s 1H c 3.8 1.5 3H s CH3 no H’s, oxygen b 3.6 1 2H s CH2 no H’s, oxygen a 2.1 1.5 3H s CH3 no H’s, carbonyl The end pieces here must be CH3’s. signals “c” and “b” are next to an O (on either side). signal “a” is probably next to a carbonyl (2.1 ppm) rather than directly off the benzene ring (2.3 ppm). C-NMR: There is a solvent signal at 77 ppm. Other than that, the C-NMR has 8 signals: one for each kind of carbon. That tells us that 2 C’s are duplicates. There is one C in the 150-200 ppm range (carbonyl C), 4 signals in the 100-150 range (the 6 benzene C’s, including the 2 duplicates mentioned in the previous sentence), 2 signals in the 50-80 range (C’s on either side of the ether O) and one signal less than 50 ppm (methyl at the end of molecule not next to an O). O O Page 2: Index of H deficiency for C9H11NO2: (21-11)/2 = 5 degrees “unsaturation” again. Probably a benzene ring, which can be confirmed by the signals near 7 ppm on the H-NMR. IR: At 1708 cm-1 is a carbonyl (C=O) again. Also visible are the two peaks of an –NH2 near 3300-3500 cm-1. sp2 and sp3 C-H’s show up again in this IR as well. H-NMR: signal ch. shift int. split what next to g 7.4 1H d benz. H 1H f 7.3 1H s benz. H 0H e 7.2 1H t benz. H 2H d 6.8 1H d benz. H 1H c 4.4 2H q CH2 3H’s CH3 & O b 3.8 2H s (broad) NH2 a 1.4 3H t CH3 2H’s CH2 One can see that the CH2 and CH3 must be together to make an ethyl. This is one of our end pieces. The other end piece is the NH2. In the middle go the carbonyl, benzene ring, and the O of the ether or ester. From the splitting pattern on the benzene ring (1 singlet, 4 signals, etc), this ring must be 1,3 substituted (called “meta” – a term we will learn in the aromatic chapter). From the chemical shift of the CH2, it must be next to the O. Given our knowledge, we can come up with two possibilities. With more experience, we might be able to differentiate between the amide and the amine, but that isn’t necessary right now. The IR carbonyl absorption is actually a little closer to an ester than an amide, but either answer is full credit for now. O O H2N O H2N or O Page 3. Index of H deficiency on C5H10O → 1 ring, alkene, or carbonyl IR: 1716 cm-1 peak indicates carbonyl. all C-H’s are sp3 H-NMR: signal chem shift split int. 10 H’s what next to a 2.4 ppm q 1 4H’s 2 CH2’s a CH3 and a C=O b 1.1 t 1.5 6H’s 2CH3’s a CH2 O IGNORE this as we have not done mass spectrometry yet. The mass spec is practically superfluous for solving the problem but does make good independent confirmation. The base peak at 57 is a loss of 29, which is the ethyl coming off one side of the carbonyl (α carbon breaks off). The peak at 29 is the ethyl coming off and keeping the positive charge. Page 4a Index of H deficiency on C9H9BrO2: (20-10)/2 = 5° again. Benzene ring confirmed by chemical shift. IR signal at 1725 cm-1 takes our last unsaturation as a C=O Top H-NMR isotope signal a chem shift 8.2 split s int 1H what benz H next to 0H b 7.9 d 1H benz H 1H c 7.7 d 1H benz H 1H d 7.3 t 1H benz H 2H e 4.4 q 2H CH2 CH3 and O f 1.4 t 3H CH3 CH2 From the splitting pattern, this is again 1,3 substituted (meta) benzene ring. End pieces are the bromine and the ethyl. In the middle, we have the benzene ring, a carbonyl and an O from an ether or ester. Given the chemical shift of the CH2, the Oxygen has to go next to it. Slightly advanced topic for consideration: the carbonyl could go between the Br and the benzene (producing an ether on the other side of the ring) or between the oxygen and the benzene (producing an ester). Given the large chemical shift (above 8 ppm), we must have two electron withdrawing groups on the benzene ring (C=O and Br) rather than one withdrawing (C=O) and one donating (O from the ether). The structure must be: O O Br Page 4a Bottom NMR isotope: signal a chem shift 7.9 split d int 2H what benz H next to 1H b 7.6 d 2H benz H 1H c 4.4 q 2H CH2 CH3 and O d 1.4 t 3H CH3 CH2 Here is the same molecule again except for the benzene H pattern. the two doublets worth 2 H’s (integration) each tells us that this benzene ring is para substituted (1,4) O Br O Page 5a Index of H deficiency (IHD) for C8H7BrO: (18-8)/2 = 5° benzene ring confirmed by signals with chemical shift at 7-8 ppm IR strong absorption bands at ~1700 on both isotopes tell that this molecule has a carbonyl, using the last double bond/ring of the IHD H-NMR for top isotope: signal a chem shift 7.6 split d int 1H what benzene H next to 1H b 7.5 d 1H benzene H 1H c 7.4 m 1H benzene H several H’s d 7.3 m 1H benzene H several H’s f* 2.6 s 3H CH3 0 H’s & aromatic *my drawing accidently has no “e,” so I have made the table match the molecule below The aromatic splitting pattern is consistent with a 1,2 disubstitution (ortho) pattern. It is clearly not a doublet of doublets (1,4 substitution) nor is it a s, d, d, m pattern that would indicate 1,3 substitution. The end pieces are the bromine and the methyl. In the middle go the benzene ring and the carbonyl. Given the chemical shift of the methyl, it is more likely to be attached to the benzene ring than next to the carbonyl (2.6 ppm closer to 2.3 than 2.1 ppm). Thus: a c O d Br b f Page 5b H-NMR for bottom isotope: signal a chem shift 7.8 split d int 2H what benzene H next to 1H b 7.6 d 2H benzene H 1H c 2.6 s 3H CH3 0 H’s & aromatic C This is eerily similar to above isotope. The only difference being the benzene ring splitting pattern. This isotope has the doublet of doublets pattern that is consistent with 1,4 substitution. a b O c Br a b