Dear Notetaker:

BHS116 Fall: Human Physiology and Pathology
Notetaker: Jessica Du
Date: 08/31/2011, 1 st hour
Page1
Exam: Friday September 2 nd (8:00 – 8:50 am)
~30-35 multiple choice questions
~ 3-4 questions/lecture
Mixture of knowledge and integration questions
Know big picture concepts
No number crunching required, but must know how equations work
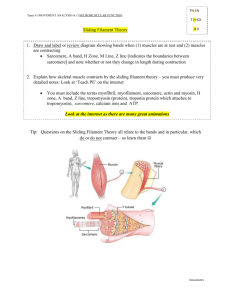
Muscle Contraction
Structure
Sarcomere
H-zone
I-band
Length during contraction
Shortens
Shortens
Shortens
Notes
2 z-disks are brought closer together
Since longer segments of the thin filament overlaps with the thick filaments during a contraction, the H zone (part of the A band that is thick filament only) decreases in length
The thin filaments of the I-band overlaps with the thick filaments
A-band Stays the same
Thick filament:
Components : Myosin protein
Structure : Functional subunit is comprised of 6 peptide chains (2 heavy chains, 4 light chains) o Tail: 2 helical intertwining heavy chains o Head: globular portion of each heavy chain interacts with 2 light chains (.`. 4 light chains in total)
The myosin head region (S1): o Participates in the formation of cross bridges with the think filament o Contributes to the sliding mechanism o ATPase : ATP
ADP + Pi o Actin-binding site: binds the thin filament o Retains all of the motor functions of myosin, used in experimental biological motor research
Each thick filament is made up of 200 or more myosin molecules (intertwining of many individual myosin tail regions)
Thin Filament:
Components (3) o 1 . F-actin : the back bone of the thin filament consisting of 2 intertwined strands in a double helix
Composed of many repeating globular subunits, when associate together, create many myosin-binding sites o 2. Tropomyosin : wraps spirally around the sides of the F-actin filaments. Blocks the myosinbinding sites on the F-actin
Since there are two strands of F-actin, there are also two strands of tropomyosin (lies in the grooves for the myosin-binding sites) o 3. Troponin Complex : complex of 3 protein subunits
A. Troponin I: strong affinity for actin
BHS116 Fall: Human Physiology and Pathology
Notetaker: Jessica Du
Date: 08/31/2011, 1 st hour
Page2
B. Troponin T: strong affinity for tropomyosin
C. Troponin C: strong affinity for Ca 2+
Walk-along mechanism of muscle contraction (aka: cross bridge cycling)
Before contraction :
1.
Myosin head binds ATP
2.
The ATPase component of the myosin head cleaves ATP into ADP + Pi (stores energy in the head)
3.
Upon ATP cleavage, the myosin head is extended toward the actin fiber, but does not attach (due to the obstruction by the tropomyosin)
Upon stimulation :
1.
The action potential reaches the neuromuscular junction, and causes the sarcoplasmic reticulum to release Ca 2+ into the myocyte
2.
Ca 2+ binds the troponin-tropomyosin complex (at the Troponin C) on the thin filament, which causes a conformational change of the complex, thus exposing the myosin-binding site of actin
3.
Myosin head can now bind to the myosin-binding site of actin (forms a cross bridge)
Power Stroke :
The cross bridge formed between the myosin head and the actin causes a conformation change to occur on the head of the myosin. This causes the head to tilt, which causes a pull on the actin by the myosin head.
Release of ADP and Pi :
After the power stroke occurs, the ADP and Pi are released from the myosin head. The myosin head will stay bound to the actin, until another molecule of ATP comes and binds.
NOTE : This is why bodies undergo rigor mortis after death. When someone dies, ATP production stop. If no ATP molecules bind the myosin head in the muscle, the muscle will stay contracted, resulting in rigor mortis. After a while, enzymes will break down the myosin protein in the corpse
– leading to the relaxation of the muscles.
The cycle of myosin head binding and being released continues until: o 1. The actin filaments pull the Z disks up against the ends of the myosin filaments o 2. The load on the muscle becomes too great for further pulling
This process occurs in 3D, with 1 centrally located thick filament, surrounded by 6 thin filaments
At the end of the muscle contraction, Ca2+ is pumped back into the sarcoplasmic reticulum
Length-Tension Relationship of INDIVIDUAL Sacromeres:
Maximum tension is developed when there is an optimum overlap of thin and thick filaments, so that all cross bridges can participate in the contraction o This occurs at the resting length of sacromeres
BHS116 Fall: Human Physiology and Pathology
Notetaker: Jessica Du
Date: 08/31/2011, 1 st hour
Page3
*Note that the physiological range of sacromere stretch/compression is 70%-130% the resting length
(A) = Resting length: optimal cross bridging, maximum tension possible
(B) = Stretched length: if the muscle is contracted at this increased muscle length, the maximal tension is lower. This is because the amount of cross bridging is reduced between the myosin/actin.
(D) = Compressed length: if the muscle of shortened, the overlapping thin filaments from opposite ends of the scarcomere interfere and conflict with each other. This restricts productive cross bridging building and reduces the maximum tension
(C) = Overstretched length: The thin filaments are pulled almost to the ends of the thick filament.
Little cross bridging is able to occur, which leads to a very low maximum tension
[ Concept : ↓ maximum tension, ↓ force of contraction ]
Resting (Passive Tension):
The tension in resting (non-contracting, eg: tendons, nerves) muscles.
It increases as the muscle is stretched to greater than normal length
Due to the elastic forces in connective tissue
Active Tension:
The tension of muscle tissue during contraction
Proportional to the number of cross bridges formed between the thick and thin filaments (follows the sacromere length curve) o Decreases as a muscle is stretched beyond its normal length
Total Tension: Passive Tension + Active Tension
At a fully stretched position, the passive and active tensions cancel each other out
If passive tension > active tension, total tension will increase