PRECIPITATION REACTIONS AND SOLUBILITY GUIDELINES
advertisement

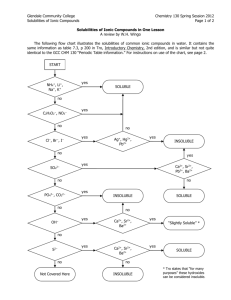
PRECIPITATION REACTIONS AND SOLUBILITY GUIDELINES Typically, chemical reactions are done in a solvent, water is often used. It is helpful to chemist to have a little idea of what to expect from a chemical reaction…and solubility rules are one tool chemist use to predict what the products are going to be in a reaction. SOLUBILITY RULES When applying solubility rules focus mainly on the anion portion of your molecule, and remember that Group 1 metals, ammonium are Always soluble. Always Soluble 1. Group 1 and ammonium (NH4+) are soluble 2. Nitrates (NO3-), acetates (CH3COO-) and perchlorates (ClO4-) are soluble 3. Cl-, Br-, and I- are soluble except with Ag+, Pb2+, Cu+ and Hg24. Sulfates (SO42-) are soluble except with Ca2+, Sr2+, Ba2+, and Pb2+ Always Insoluble 5. CO32- are Insoluble expect with Group 1 and NH4+ 6. PO43- are Insoluble expect with Group 1 and NH4+ 7. S2- are Insoluble expect with Group 1, 2 and NH4+ 8. OH- are Insoluble except with Group 1, Ca2+, Sr2+, Ba2+ For instance determine if LiCl, K2S and Mg(OH)2 are soluble ionic compounds in Water: 1. LiCl: Rule 1 says all group 1 salts are ALWAYS soluble, so we would write this as: LiCl(s) Li+ (aq) + Cl- (aq) 2. K2S: Same as above rule 1 and rule 7 both say this will be soluble. This would be written as soluble products in water as: K2S(s) 2K+ (aq) + S-2 (aq) Note: to break apart the ionic compound into it’s original ionic “formal” units. 3. Mg(OH)2: Rule 8 says this ionic molecule will be Insoluble in water, or it will form a precipitate as: Mg(OH)2 (s) Mg(OH)2 (s) and will not dissociate in water. 1 Determine the solubility of each molecule in the following chemical reactions, specify using (aq) = aqueous which means soluble in water, (g) = gas, (s) = solid and (l) = water or solvent. 1. 2HCl + Ba(OH)2 2. Na2SO4 BaCl2 + 2H2O + Sr(NO3)2 SrSO4 + 2NaNO3 Did you get the following: 1. 2HCl(aq) + Ba(OH)2(aq) BaCl2(aq) + 2H2O(l) 2. Na2SO4(s) + Sr(NO3)2(s) SrSO4(s) + 2NaNO3(aq) Important to the topic of Solubility is that sometimes products of chemical reactions decompose further, there are 3 such reactions that we must always watch for because they will always produce a gas. Gas-Forming Reactions Notice the formation of the following products decompose into a gas and water 1. H 2 CO 3 CO 2 (g) + H 2 O(l) 2. H 2 SO 3 SO 2 (g) + H 2 O(l) 3. NH 4 OH NH 3 (g) + H 2 O(l) Also, the formation of 4. H 2 O as a product is always a Liquid (l) 5. H 2 S is always written as a gas or H 2 S (g) Examples: 1 & 4: Na 2 CO 3 (aq) + 2 HCl(aq) 2 NaCl(aq) + CO 2 (g) + H 2 O(l) 2 & 4: Na 2 SO 3 (aq) + 2 HCl(aq) NaCl(aq) + SO 2 (g) + H 2 O(l) 2 3 & 4: NH 4 Cl(aq) + NaOH(aq) NaCl(aq) + NH 3 (g) + H 2 O(l) 5. Na 2 S(aq) + 2 HCl(aq) 2NaCl(aq) + H 2 S(g) Look at the following reaction and determine the products for this double replacement reaction. HCl(aq) + Ag2CO3(s) Did you write the following? 2HCl(aq) + Ag2CO3(s) 2AgCl(s) + H2O(l) + CO2(g) Notice the formation of CO2 (g) this is an example of one of the 3 special gas forming reactions. Putting together what we’ve learned to this point will help us write the formula we always try to determine in chemistry---the Net Ionic Equation or NIE for short. Net Ionic Equations tell us what takes place in a chemical reaction. There are always 3 steps in determining the NIE. The first step is to write the Molecular Equation. The second is to write the Total Ionic Equation. The third is to write the NIE. Here is an example illustrated with sodium hydroxide and cadmium nitrate. Let us write out the first equation, often the HARDEST equation because we must determine 1. The products (do not forget to look for one of three special gas-forming reactions), 2. the balance coefficients for each molecule, and 3. the state of each molecule whether it is a solid, liquid or gas. 1. Molecular Equation (refer to Solubility Rules): 2NaOH (aq) + Cd(NO3)2(aq) Cd(OH)2(s) + 2NaNO3(aq) In the second equation, the Total Ionic Equation, we look at the previous Molecular Equation and we notice (aq) and (s) states. To write the Total Ionic we find the (aq) molecules and break those apart into formal units. If the molecule has a (s) then we leave it alone. 3 2. Total Ionic Equation---molecular equation broken into ions. 2Na+(aq) + 2OH-(aq)+ Cd2+(aq)+ 2NO-3(aq) Cd(OH)2(s) + 2Na+(aq) + 2NO-3(aq) Important: notice the coefficients in front of all the ions. We need to have he same number of elements on each side of the equation. For the last equation, we look at equation 2. Total Ionic and look for anions, cations, atoms that do NOT appear on each side of the arrow and write those down. 3. Net Ionic---what made the reaction and it’s starting ions. 2OH-(aq)+ Cd2+(aq) Cd(OH)2(s) Write the 1. Molecular, 2. Total and 3. Net Ionic Equations for: Iron (III) chloride + cesium phosphate Iron (III) phosphate + cesium chloride 4