Lecture 2
advertisement

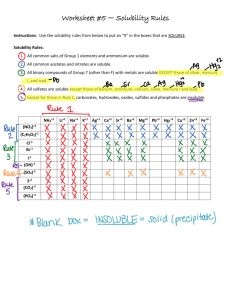
Course Logistics • Textbook – An online option is available for the Campbell and Farrell text • Problem Sets – The problem sets will consist of problems very similar to those assigned on the class schedule – They are due on the date posted on the class schedule • CHEM108 – The lab course is a corequisite and you must pass the lab class to receive a grade for CHEM106 • Chemistry tutors – They should start next week • Amino Acids – Start learning them now. – By the end of next week, you should know the chemical structure of: A, V, S, D, E, R, K, C, H, F Law of Conservation of Matter • “Matter can neither be created nor destroyed” – Antoine Lavoisier, 1774 If a complete chemical reaction has occurred, all of the reactant atoms must be present in the product(s) Law of Conservation of Matter a) b) •Stoichiometric coefficients are necessary to balance the equation so that the Law of Conservation of Matter is not violated •6 molecules of Cl2 react with 1 molecule of P4 •3 molecules of Cl2 react with 2 molecules of Fe Example of Using Stoichiometric Coefficients Balancing Chemical Reactions • Let’s look at Oxide Formation • Metals/Nonmetals may react with oxygen to form an oxide with the formula MxOy • Example 1: Iron reacts with oxygen to give Iron (III) Oxide Fe (s) + O2 (g) → Fe2O3 (s) How do we solve it? Fe (s) + O2 (g) → Fe2O3 (s) • Step 1: Look at the product. There are 3 atoms of oxygen in the product, but we start with an even number of oxygen atoms. – Let’s convert the # of oxygens in the product to an even number Result: Fe (s) + O2 (g) → 2Fe2O3 (s) How do we Solve It? Fe (s) + O2 (g) → 2Fe2O3 (s) • Then, balance the reactant side and make sure the number/type of atoms on each side balance. Balanced Equation: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) How do we Solve It? • Example 2: Sulfur and oxygen react to form sulfur dioxide. S (s) + O2 (g) → SO2 (g) • Step 1: Look at the reaction. We lucked out! Balanced Equation: S (s) + O2 (g) → SO2 (g) How do we Solve It? • Example 3: Phosphorus (P4) reacts with oxygen to give tetraphosphorus decaoxide. P4 (s) + O2 (g) → P4O10 (s) • Step 1: Look at the reaction. The phosphorus atoms are balanced, so let’s balance the oxygens. Balanced Equation: P4 (s) + 5O2 (g) → P4O10 (g) How do we Solve It? • Example 4: Combustion of Octane (C8H18). C8H18 (l) + O2 (g) → CO2 (g) + H2O (g) • Step 1: Look at the reaction. Then: – Balance the Carbons C8H18 (l) + O2 (g) → 8CO2 (g) + H2O (g) How do we Solve It? C8H18 (l) + O2 (g) → 8CO2 (g) + H2O (g) • Step 2: Balance the Hydrogens C8H18 (l) + O2 (g) → 8CO2 (g) + 9H2O (g) • Step 3: Balance the Oxygens – Problem! Odd number of oxygen atoms • 12.5 Oxygens on reactant side – Solution: Double EVERY coefficient (even those with a value of ‘1’) How do we Solve It? C8H18 (l) + 12.5O2 (g) → 8CO2 (g) + 9H2O (g) • Step 3 (cont’d): Balance the Oxygens 2C8H18 (l) + 25O2 (g) → 16CO2 (g) + 18H2O (g) • Step 4: Make sure everything checks out Review of Balancing Equations Aqueous Solutions and Precipitation Reactions Terminology: 1. Soluble substance: A substance that dissolves to a significant extent in a specific solvent • Electrolytes: Strong acid, strong base, soluble ionic compounds 2. Insoluble substance: A substance that does not dissolve significantly in a specific solvent Soluble K2CrO4 (yellow) Soluble AgNO3 (clear) Insoluble Ag2CrO4 (red) Electrolytes • Remember that when dealing with strong acids and strong bases, the two will react to make a salt and water – Strong acids: • Any hydrohalogen acid • Nitric, Chloric and sulfuric acids – Strong bases: • Group I hydroxides • Group 2, Period 3 or higher hydroxides • Group 1/Group 2 Oxides • To determine how much strong acid or base to add necessary to neutralize a strong base or acid, you’ll need to know what? Precipitation Reactions • In a Precipitation Reaction, an insoluble product forms when we mix two electrolyte solutions • What determines if something precipitates when the solutions are mixed? – Intermolecular forces between the solute and solvent – The entropy change that occurs as a result of solvation If we mix the soluble solutions NaCl (aq) and AgNO3 (aq) How do we determine if a precipitate forms? Step 1: Write the balanced chemical equation for the double displacement reaction AB + CD --> AD + BC • Remember the charges on the ions Step 2: Using the Solubility rules, determine if either product is insoluble – If all products are insoluble, then no reaction occurs Step 3: Write the Complete and Net Ionic equations for the reaction Complete and Net Ionic Equations • A Complete Ionic Equation shows all chemical species present in the reaction • A Net Ionic Equation shows the net change taking place in the reaction – The Net Ionic Equation is made by taking the Spectator Ions out of the complete ionic equation Solubility Equilibria • When we put an ionic compound in a solvent, even if it is considered to be insoluble, some small number of ions dissolve into the bulk phase • For an ionic solid of formula AB, this can be expressed as the equilibrium reaction: AB (s) A+ (aq) + B- (aq) Solubility Product • The equilibrium constant for the solubility equilibrium of an ionic solid is called the solubility product, Ksp AB(s) mA+ (aq) + nB- (aq) Ksp = [A+]m [B-]n We don’t put [AB] in the denominator, why? Solubility Product •The smaller the value of Ksp, the less soluble an ionic solid is •We calculate Ksp using Molar Solubility •Molar concentration of the compound in a saturated solution Solubility Product • We can use the solubility products and molar solubilities to predict how precipitation will proceed • Remember: Low Ksp, Low Solubility The Common Ion Effect We can use Le Chatelier’s Principle to find a way to remove ions from solution – Application: Heavy metal cleanup CdCO3 Cd2+ + CO32If we add potassium carbonate to the solution, what will happen? The decrease in CdCO3 soubility caused by addition of another carbonate salt is an example of The Common Ion Effect