Ionic Compound Formulas and Naming for Multivalent Metals Notes
advertisement

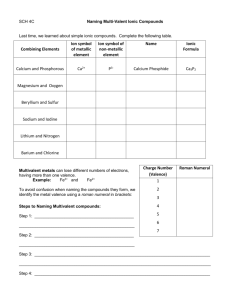
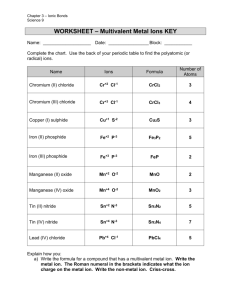
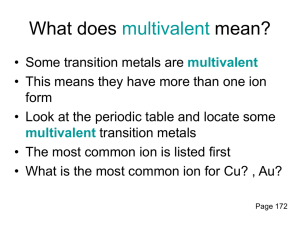
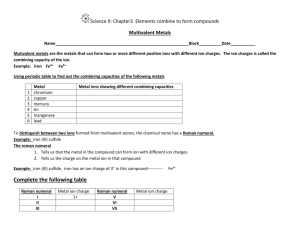
Ionic Compound Formulas and Naming for Multivalent Metals Notes What does multivalent mean? Some transition metals are multivalent • This means they have more than one ion form • • Look at the periodic table (textbook) and locate some multivalent transition metals The most common ion is listed first • What is the most common ion for Cu? , Au? Writing names for multivalent ions •We need to distinguish between the two or three different ion forms •Use roman numerals from I – VII which corresponds to 1+ - 7+ ion charges •For example: –Nickel can have two ion forms, Ni2+ and Ni3+ –These are named nickel (II) and Nickel (III) Writing formulas for compounds containing a multivalent metal •There will only be 1 multivalent metal in a compound •It is the metal so it is first in the name (same as ionic compounds) •The roman numeral will indicate which ion form is in the compound ***If you are not told what the charge is on the metal with more than one charge, assume it is the most common charge. (charge on top)*** Steps for writing formulas for multivalent compounds •Step 1: Identify each ion and its charge •Step 2: Determine the total charges needed to balance positive and negative •Step 3: Note the ratio of positive ions to negative ions •Step 4: Use subscripts to write the formula, 1’s are not shown in subscript Try the formula for titanium (IV) fluoride manganese (III) sulfide Compound Naming To name metals with more than one charge we use the Stock system. In the Stock system, the charge of the metal is indicated by a Roman numeral written after the name of the metal. o Fe2O3 - iron(III) oxide o FeO - iron(II) oxide o PbO2 - lead(IV) oxide The Stock system should not be used for metals that have only one possible charge. Steps for Writing names for formulas for compounds that contain a multivalent metal (stock system) •Step 1: Identify a multivalent metal •Step 2: identify its different ion forms •Step 3: determine the ratio of ions in the formula •Step 4: what is the charge on the negative ion? •Step 5: Balance the positive and negative charges •Step 6: Write the name using roman numerals in brackets, following the positive ion to indicate which ion was used Example: FeI2 Example: PbF4