Ionic compound with a multivalent metal
advertisement
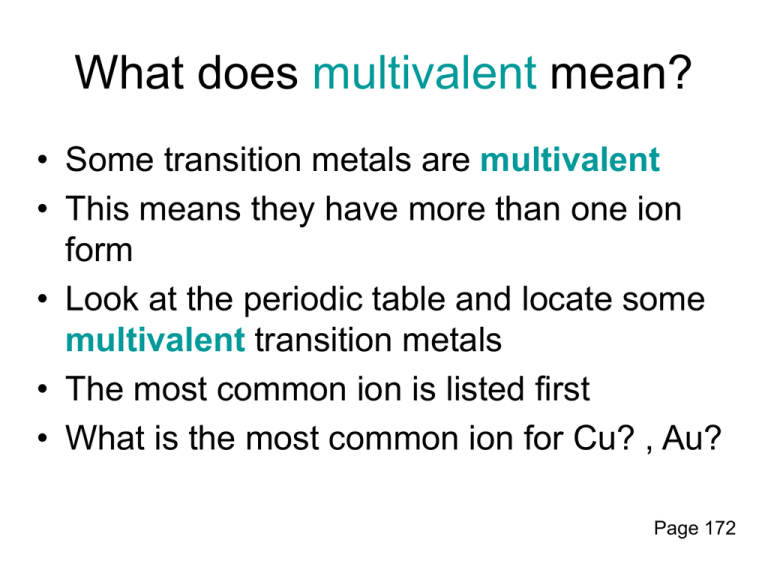
What does multivalent mean? • Some transition metals are multivalent • This means they have more than one ion form • Look at the periodic table and locate some multivalent transition metals • The most common ion is listed first • What is the most common ion for Cu? , Au? Page 172 What are multivalent ions? For Example: Copper has 2 ion forms Can be a 1+ or 2+ ion Copper I Chloride Copper II Chloride Writing names for multivalent ions • We need to distinguish between the two or three different ion forms • Use roman numerals from I – VII which corresponds to 1+ - 7+ ion charges Metal Ion charge 1+ 2+ 3+ 4+ 5+ 6+ 7+ Roman Numeral I II III IV V VI VII • For example: – Nickel can have two ion forms, Ni2+ and Ni3+ – These are named nickel (II) and Nickel (III) Writing formulas for compounds containing a multivalent metal • There will only be 1 multivalent metal in a compound • It is the metal so it is first in the name (same as ionic compounds) • The roman numeral will indicate which ion form is in the compound Steps for writing formulas for multivalent compounds • Step 1: Identify each ion and its charge • Step 2: Determine the total charges needed to balance positive and negative • Step 3: Note the ratio of positive ions to negative ions • Step 4: Use subscripts to write the formula, 1’s are not shown in subscript Try the formula for titanium (IV) fluoride ions Total charge to balance Ti4+ and F1+4 = -1-1-1-1 ratio 1:4 formula TiF4 189-191 Lowest Common Multiple-A useful Trick Manganese (III) Sulfide Lowest common multiple for 3 and 2 is 6 ions Total charge to balance 3+ Mn and 2S +3+3 = -2-2-2 ratio 2:3 formula Mn2S3 Writing formulas for compounds that contain a multivalent metal Key Points: • The metal is always first • The metal is always the positive ion • The metal could be a multivalent metal • Go to the periodic table and check Steps for Writing formulas for compounds that contain a multivalent metal • • • • • Step 1: Identify a multivalent metal Step 2: identify its different ion forms Step 3: determine the ratio of ions in the formula Step 4: what is the charge on the negative ion? Step 5: Balance the positive and negative charges • Step 6: Write the name using roman numerals in brackets, following the positive ion to indicate which ion was used Example: FeI2 • Is there a multivalent metal? • Yes, Fe, Iron • What are its different ion forms? • 3+ and 2+ • What is the ratio of ions • 1 Fe : 2 I • What is the charge on the negative ion • I, Iodine, ion charge is 1• but there are 2 so the total negative charge is 2• Balance the positive and negative charges • Iron must have a 2+ charge to balance the two 1- charges from Iodine • Write the name using a roman numeral to say which ion form of Iron is present • The negative ion must drop its ending and add –ide (same as ionic compounds) • Iron (II) Iodide Try another example: PbF4 • • • • • Pb 2+ and 4+ Pb 1: 4 F F’s ion charge 1Balance the charges • 4+ charges to balance the 4 1- charges of F • Therefore Pb must be the 4+ ion • Lead (IV) Fluoride Meet out new class plant! Video Homework to help you practice writing formulas and naming compounds that contain a multivalent metal • In workbook p. 68 1-3 Questions to practice writing formulas for ionic compounds Text book p. 188 1. a, c, e 2. b, d, f, h, j, l, n