cl bonds
advertisement

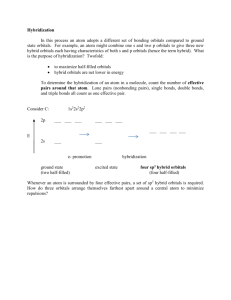
1 B. H2S 1. Determine Lewis structure: H S H 2. Count number of electron domains around the central atom. S is the central atom. Four electron domains, therefore electron domain geometry is tetrahedral. 3. From the electron domain geometry, identify the molecular geometry by considering the bonded atoms. Two lone pairs and two bonding domains, therefore molecular geometry is bent. S H H 2 2.4. Hybridisation Chap. 9, Section 9.5 p.361-367 In predicting structures using VSEPR, we have considered bonding as sharing of electron pairs. What about bonding in terms of atomic orbital overlap? Example: BeCl2 z y Cl Be Cl x Linear with two identical Be-Cl bonds. Be: [He] 2s22p0 Cl:[Ne]3s23p5. In order to understand how Be can share its 2 electrons, we can consider an alternative electron configuration: [He]2s12p1. (Costs energy). 3 Then the two bonds are formed by overlap of (a) 2s on Be and 3py on Cl and (b) 2py on Be and 3py on Cl. Gives two bonds each having a different energy! Solution: Mix 2s and the 2py atomic orbitals on Be before forming the bonds. See diagram. This gives two new orbitals. This mixing is known as hybridisation; the new orbitals formed are sp hybrid orbitals; the Be is said to be sp hybridised. Note that each of the sp hybrid orbitals points along the y axis, thus they are in the right orientation to form a linear compound. 4 (A) z z y + x (B) y x z y x z y - x y x z z z y x z + y y x x 5 A similar approach is used to explain the bonding in compounds with other shapes. Example: BCl3 Trigonal planar (check!) There are three B-Cl bonds. Thus there are three orbitals on B involved in bonding. These three orbitals are derived from the mixing or hybridisation of three atomic orbitals on B, i.e. the 2s, and two of the 2p a.o.’s (2py and 2pz). The hybrid orbitals are sp2 hybrid orbitals and B is said to be sp2 hybridised. The lobes of the sp2 hybrid orbitals lie in the correct orientation to form a trigonal planar compound. Note. No. of hybrid orbitals on a bonded atom = No. of a.o.s used to form the hybrid orbitals 6 What about compounds with one or more lone pairs of electrons? e.g. :NH3. Must include the lone pair when considering the number of hybrid orbitals required. N in NH3 has four electron domains, therefore there are four hybrid orbitals on N in NH3. Four hybrid orbitals are formed by mixing four atomic orbitals, here 2s and three 2p’s. Hybridisation involved is sp3. In general, the type of hybridisation depends on the electron domain geometry of the molecule or ion (VSEPR). No. of electron domains around central atom = no. of hybrid orbitals on central atom 7 To assign hybridisation in a compound: (i) Determine Lewis structure of molecule or ion (ii) Determine electron domain geometry (iii) Assign set of hybridised orbitals that correspond to this geometry. Question Give the hybridisation of the central atom in (i) PCl5 (ii) H2S. 8 (Use Lewis structures determined previously) (i) Cl Cl Cl Cl P Cl Five electron domains around P, therefore electron domain geometry is trigonal bipyramidal. Need five hybrid orbitals on P. Formed by mixing five a.o’s i.e. one 3s, three 3p, and one d a.o. Hybridisation is sp3d.