Palladium, Gold and Gold-palladium Nanoparticle Supported
advertisement

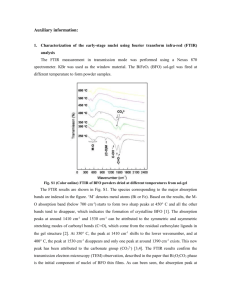
Palladium, Gold and Gold-palladium Nanoparticle Supported Carbon Materials for Cyclohexane Oxidation VISHAL J. MAYANI, SURANJANA V. MAYANI AND SANG WOOK KIM* Department of Advanced Materials Chemistry, College of Science and Technology, Dongguk University, Gyeongju, Gyeongbuk 780-714, Republic of Korea, Email: swkim@dongguk.ac.kr, Tel: +82-54-770-2216, Fax: +82-54-770-2386 Synthesis of Nano Silica Ball (NSB-25/170). Nano silica ball (NSB) was prepared by hydrolysis and condensation of TEOS. Here, we have synthesized two sets of NSB (25 and 170 nm). In a typical synthesis of NSB-25, 10 mL distilled water and 7.8 mL of TEOS were stirred (450 rpm) in 500 mL of absolute ethanol (EtOH) for 30 min. 4 mL of NH4OH (28 wt %) was added to the resulting mixture, which was left to stir for further 24 h at room temperature at 450 rpm. The reaction mixture was transferred to oven and heated at 70 °C until complete dryness. The solid product was washed (ethanol and warm water) and dried at room temperature in air. The resulted NSB-25 was calcined at 550 °C for 6 h. For NSB-170, 10.5 mL distilled water and 30 mL of TEOS were stirred in 500 mL of ethanol (EtOH) for 30 min. 14 mL of NH4OH (28 wt %) was added to the resulting mixture, which was left to stir for further 4-5 h at room temperature at 450 rpm. The reaction mixture was transferred to oven and heated at 70 °C until complete dryness. The solid product was washed (ethanol and warm water) and dried at room temperature in air. The resulted NSB-170 was calcined at 550 °C for 6 h. (NSB-25); yield (2.3 g), BET Surface area of NSB-25: 30 m2/g, FTIR (KBr) 811, 1104, 1627, 1870, 3439, 3746 cm-1. (NSB-170); yield (6.1 g), FTIR (KBr) 469, 553, 799, 949, 1096, 1396, 1632, 3430 cm-1, BET Surface area of NSB-170: 163 m2/g (Mayani et al., 2012 a). Synthesis of Carbon Cage (CC-25/170). The pitch residue, used as a carbon cage precursor, was prepared according to the method reported previously under a heat extraction and selfcrystallization method (Mayani et al., 2011). For a typical procedure, 12 g of pitch was heated at 50 °C and mixed homogeneously with 10 g of NSB-25/NSB-170 in crucible for 10 min. After cooling to room temperature, the mixture was carbonized at 900 °C for 4 h under nitrogen atmosphere to produce the carbon silica composite (CSC-25/170) material. The resulting CSC25/170 material was treated with 10 % hydrofluoric acid (HF) for 12 h. The solid carbon cage (CC-25/170) material was washed with distilled water and followed by drying at 100 °C in oven (Scheme 1). (CC-25); yield (6.3 g), BET Surface area of CC-25: 82 m2/g, FTIR (KBr) 1113, 1584, 1620, 3437 cm-1. Elemental analysis results showed that CC-25 contained > 94 wt % carbon and < 1.2 wt % H after carbonization at > 900 °C. (CC-170); yield (6.2 g), BET Surface area of CC-170: 212 m2/g, FTIR (KBr) 811, 1099, 1257, 1602, 3453 cm-1. Elemental analysis results showed that CC-170 contained > 95 wt % carbon and < 0.4 wt % H after carbonization at > 900 °C (Mayani et al., 2012 b). Characterization. The synthesized palladium and gold-palladium incorporated carbon nanocomposites were fully characterized by microanalysis, N2 adsorption–desorption isotherm, SEM–EDS, TEM, ICP, TGA, and PXRD (Figures S1 to S11). Relative Intensity (a.u.) (e) (d) (c) (b) (a) 0 2 4 6 8 10 2 theta (Degrees) Figure S1. Low-angle PXRD patterns of NSB-25 (a), CC-25 (b), CGC-25 (c), CPC-25 (d) and CGPC-25 (e). Relative Intensity (a.u.) (t) (s) (r) (q) (p) 0 2 4 6 8 10 2 theta (Degrees) Figure S2. Low-angle PXRD patterns of NSB-170 (p), CC-170 (q), CGC-170 (r), CPC-170 (s) and CGPC-170 (t). Relative Intensity (a.u.) (E) (D) (C) (B) (A) 10 20 30 40 50 60 70 80 2 theta (Degrees) Figure S3. Wide-angle PXRD patterns of NSB-25 (A), CC-25 (B), CGC-25 (C), CPC-25 (D) and CGPC-25 (E). Relative Intensity (a.u.) (T) (S) (R) (Q) (P) 10 20 30 40 50 60 70 80 2 theta (Degrees) Figure S4. Wide-angle PXRD patterns of NSB-170 (P), CC-170 (Q), CGC-170 (R), CPC-170 (S) and CGPC-170 (T). Figure S5. SEM images of NSB-25 (A), CC-25 (B), CGC-25 (C), CPC-25 (D) and CGPC-25 (E). Figure S6. SEM images of NSB-170 (a), CC-170 (b), CGC-170 (c), CPC-170 (d) and CGPC170 (e). Figure S7. TEM images of NSB-25 (A), CC-25 (B), CGC-25 (C), CPC-25 (D) and CGPC-25 (E and F). Figure S8. TEM images of NSB-170 (a), CC-170 (b), CGC-170 (c), CPC-170 (d) and CGPC170 (e). 200 140 120 Volume Adsorbed 160 140 120 Volume Adsorbed 180 100 80 60 40 20 100 0 0.0 80 NSB-25 0.2 0.4 0.6 0.8 1.0 Relative Pressure (P/Po) 60 40 20 NSB-170 0 0.0 0.2 0.4 0.6 0.8 Relative Pressure (P/Po) Figure S9. Nitrogen adsorption-desorption isotherms of NSB-25 and NSB-170 1.0 100 % Weight loss 95 90 NSB-25 CC-25 CGC-25 CPC-25 CGPC-25 85 80 0 100 200 300 400 500 600 700 800 900 Temperature/Time Figure S10. Thermo-gravimetric analysis (TGA) curves of NSB-25, CC-25, CGC-25, CPC-25 and CGPC-25. 100 % Weight loss 95 90 NSB-170 CC-170 CGC-170 CPC-170 CGPC-170 85 80 0 100 200 300 400 500 600 700 800 900 Temperature/Time Figure S11. Thermo-gravimetric analysis (TGA) curves of NSB-170, CC-170, CGC-170, CPC170 and CGPC-170. References Mayani, S. V.; Mayani, V. J.; Park, S. K.; Kim, S. W. (2012 a). Synthesis and characterization of metal incorporated composite carbon materials from pyrolysis fuel oil, Mater. Lett., 82, 120-123. Mayani, V. J., Mayani, S. V., Kim, S. W. (2012 b). Development of nanocarbon gold composite for heterogeneous catalytic oxidation, Mater. Lett, 7, 90-93. Mayani, V.J., Mayani, S.V., Lee ,Y., Park, S.K. (2011). A non-chromatographic method for the separation of highly pure naphthalene crystals from pyrolysis fuel oil, Sep. Purif. Technol., 80, 90-95.