Auxiliary_information
advertisement
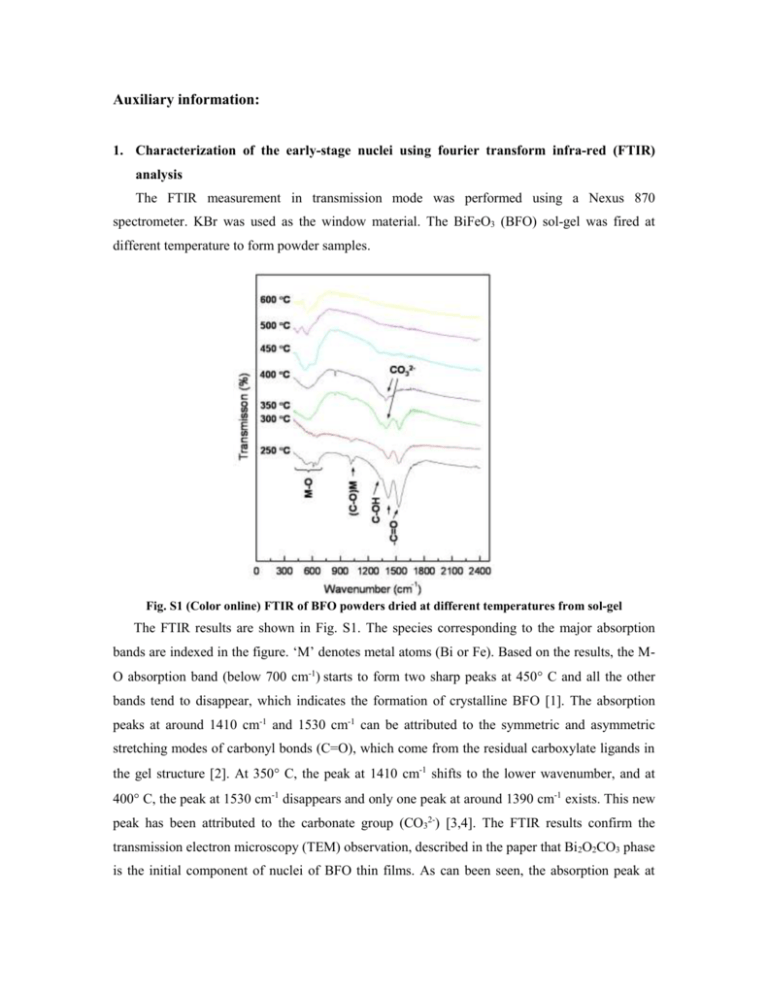
Auxiliary information: 1. Characterization of the early-stage nuclei using fourier transform infra-red (FTIR) analysis The FTIR measurement in transmission mode was performed using a Nexus 870 spectrometer. KBr was used as the window material. The BiFeO3 (BFO) sol-gel was fired at different temperature to form powder samples. Fig. S1 (Color online) FTIR of BFO powders dried at different temperatures from sol-gel The FTIR results are shown in Fig. S1. The species corresponding to the major absorption bands are indexed in the figure. ‘M’ denotes metal atoms (Bi or Fe). Based on the results, the MO absorption band (below 700 cm-1) starts to form two sharp peaks at 450 C and all the other bands tend to disappear, which indicates the formation of crystalline BFO [1]. The absorption peaks at around 1410 cm-1 and 1530 cm-1 can be attributed to the symmetric and asymmetric stretching modes of carbonyl bonds (C=O), which come from the residual carboxylate ligands in the gel structure [2]. At 350 C, the peak at 1410 cm-1 shifts to the lower wavenumber, and at 400 C, the peak at 1530 cm-1 disappears and only one peak at around 1390 cm-1 exists. This new peak has been attributed to the carbonate group (CO32-) [3,4]. The FTIR results confirm the transmission electron microscopy (TEM) observation, described in the paper that Bi2O2CO3 phase is the initial component of nuclei of BFO thin films. As can been seen, the absorption peak at 1530 cm-1 still exists at 350 C. We speculate that this is from the carbonyl bonds associated with the Fe source. Since two types of carboxylates for Bi and Fe were used, the decomposition temperature may be different. 2. Grazing-incidence small-angle x-ray scattering (GISAXS) Figure S2 shows the schematic illustration of the GISASX geometry. Incident beam is coming along –x direction. i and f are the angle of the incident x-ray beam and the exit angle of scattered beam with respect to the sample surface, respectively. 2f is the angle between the scattered beam and the plane of incidence. qy and qz are the in-plane and out-of-plane components of the scattering wave vector q, which is defined as [5,6] cos( f ) cos( 2 f ) cos( i ) qx 2 q k f ki q y cos( f ) sin( 2 f ) q sin( ) sin( ) f i z (1) where ki and kf stand for the wave vector for incident and exit beam, respectively. Since the charged-coupled device detector is positioned close to the y-z plane, qx is very small in the present case and can be ignored during simulation. Therefore, the wave vector q q y2 qz2 . Fig. S2 (Color online) The geometry of GISAXS 3. Distribution of clusters and nuclei at different temperature Qualitatively, the evolution of the initial 2D distribution of the scattering centers may be caused by either one or a combination of several reasons. First, the pre-existing clusters are driven by the evaporation of the solvent to move towards the substrate. This is analogous to the process for preparing nanoparticle arrays or crystals from their colloidal suspension [7]. Second, the stress field near the film-substrate interface can also be responsible for the 2D distribution of the scattering centers. During annealing, the film undergoes a large in-plane tensile stress because of the expansion of the substrate and the shrinkage of the film. At lower temperature, the stress is more likely to be released along the direction perpendicular to the surface, because the film is more flexible. For PZT thin films, the formation of the pyrochlore phase at lower temperatures is largely due to the larger thermal expansion coefficient, which effectively releases the stress in the film[8]. In the present case, the formation of intermediate phase Bi2O2CO3 perhaps reflects a similar mechanism. 4. Thermogravimetric analysis (TGA) of sol-gel Figure S3 shows the result of TGA, as well as the corresponding differential curve, of the BFO powder dried from the sol-gel precursor. It can be observed that two remarkable decomposition reactions take place around 190 C and 280 C, respectively. At temperatures higher than 300 C, the sample weight maintains at a certain value, which means the decomposition of long chain organic molecules is basically complete. Moreover, we did not observe crystallize phase at temperature below 300 C, therefore, the scattering centers tends to be amorphous clusters at the low temperature region, but nuclei at higher temperatures. Fig. S3 TGA curve of BFO sol-gel dried powder and corresponding differential curve References [1] D. Voll, A. Beran, H. Schneider, Phys. Chem. Miner. 33, 623 (2006). [2] S.G. Cho, P.F. Johnson, R.A. Condrate, J. Mater. Sci. 25, 4738-4744 (1990). [3] G. Braunstein, et al., J. Appl. Phys. 73, 961 (1993). [4] U. Hasenkox, S. Hoffmann, R. Waser, J. Sol-Gel. Sci. Technol. 12, 67 (1998). [5] B.D. Lee, B.D. et al., Nature Materials 4, 147 (2005). [6] C. Revenant, et al., Phys. Rev. B 69, 035411 (2004). [7] A.S. Dimitrov, K. Nagayama, Langmuir 12, 1303 (1996) [8] G. Scherer, J. Sol-Gel Sci. Techn. 8, 353 (1997)