Simple chemical reactions
advertisement

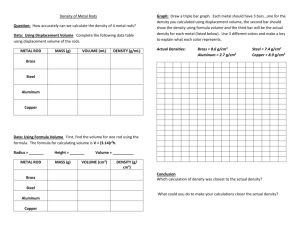
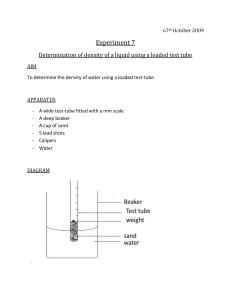
Simple Chemical Reactions 19 marks total. Aim: Simple chemical reactions e.g. reaction of a metal with an acid, reaction of a carbonate with an acid, a metal displacement reaction, precipitation reaction and thermal decomposition. Chemicals required: Copper carbonate CuCO3 (10g), sodium carbonate Na2CO3 (10g), zinc metal Zn (5g), copper(II) sulphate CuSO4 (10g), dilute hydrochloric acid HCl(aq) (250 mL), dilute nitric acid HNO3(aq) (250 mL), dilute sulphuric acid H2SO4(aq) (250 mL), copper metal Cu (5g), iron powder Fe (5g), sodium chloride NaCl (5g), silver nitrate AgNO3 (2g). Apparatus required: 10 x test tubes, 2 x test-tube holder, 1 x test-tube rack, 3 x spatula, 3 x dropping pipette, spirit burner. 1. Reactions of metals with acids Add about 1 cm3 of the dilute acid to a test-tube. Then add a small amount of the metal to the acid. Write down any observations and chemical equations. Acids are corrosive. Reaction with hydrochloric acid Metal Reaction with sulphuric acid Reaction with nitric acid Zinc Iron Copper 2. Reactions of a carbonate with an acid Add about 1 cm3 of the dilute acid to a test-tube using a dropping pipette. Then add a small amount of the metal carbonate to the acid. Write down any observations and chemical equations. 3. Metal displacement reaction Add about 2 cm3 of copper(II) sulphate solution to a test-tube using a dropping pipette. Then add a small amount of the metal. Write down any observations and chemical equations. Copper compounds are harmful. 4. Precipitation reaction Add about 2 cm3 of dilute silver nitrate solution to a test-tube using a dropping pipette (if a solution isn’t available, dissolve a small amount of silver nitrate in water). Then add a small amount of the aqueous sodium chloride solution. Write down any observations and chemical equations. 5. Thermal decomposition reaction Add a spatula of copper(II) carbonate to a test-tube. Heat the test-tube strongly for a few minutes and write down any observations. Then allow it to cool and add the remaining powder to about 2 cm3 of dilute sulphuric acid in another test-tube. Warm the mixture and write down any observations. Write down chemical equations for any reactions that occur.