reactions of acids practical IGCSE
advertisement

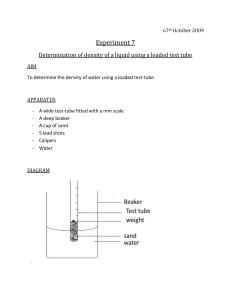
Reactions of acids In this experiment you will investigate three reactions of acids. You will use hydrochloric acid as a typical acid SAFETY SAFETY SPECS MUST BE WORN HYDROCHLORIC ACID IS CORROSIVE !! TAKE CARE WHEN HEATING LIQUIDS !! 1. Reaction with a metal carbonate (calcium carbonate) (a) Add 2 squirts of acid to a test-tube (b) Assemble your apparatus to collect a gas produced (c) Add a spatula of calcium carbonate to the test-tube and collect and test the gas produced Observations 2. Reaction with a reactive metal (magnesium) (a) Add 2 squirts of acid to a test-tube (b) Assemble your apparatus to collect a gas produced (c) Add two small pieces of magnesium to the test-tube and collect and test the gas produced Observations 3. Reaction with a soluble base (sodium hydroxide) (a) Add 2 squirts of acid to a test-tube (b) Add a few drops of universal indicator (c) Add sodium hydroxide, drop by drop, noting any colour changes Observations 4. Reaction with a solid base I (a) Add 2 squirts of acid to a small beaker (b) Add half a spatula of copper (II) oxide (c) WARM GENTLY on a LOW heat (you are not collecting any gas) Observations 5. Reaction with a solid base II Repeat the procedure for Reaction 4 using sulphuric acid instead of hydrochloric acid Observations Questions 1. Complete the following general word equations (a) Acid + metal carbonate ______ + ______ + ______ (b) Acid + metal ________ + _______ (c) Acid + base ______ + ______ 2. Write a word equation and a balanced chemical equation for the five reactions that you performed 1) 2) 3) 4) 5)