9Ec3Carbonates and acids
advertisement

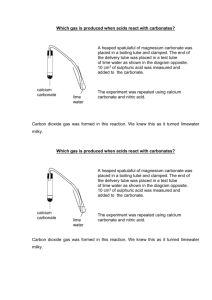
9Ec(3) Carbonates and acid How do metal carbonates react with acid? Many rocks contain carbonates. This experiment shows how carbonates react with dilute sulphuric acid. ? Apparatus • • • • • • • copper carbonate powder dilute sulphuric acid 100cm3 beaker universal indicator paper sodium carbonate powder calcium carbonate powder dilute hydrochloric acid • • • • • • • boiling tubes bung delivery tube spatula limewater dilute nitric acid forceps Wear eye protection. Keep away from any spray from the reactions. Nitric acid is an irritant at the concentration supplied. Method A Carefully put 10cm3 of dilute sulphuric acid in a 100cm3 beaker. Holding indicator paper using forceps, measure the pH of the acid. B Add one spatula of powdered copper carbonate. Be careful to keep away from any spray caused by the reaction that takes place. Record your observations. C Add some more copper carbonate and look carefully at the mixture. Keep adding small amounts of copper carbonate until there is no further reaction. D Does the test tube get warmer? E Test the pH of the final solution with indicator paper. F Repeat your experiment using either a different metal carbonate instead of copper carbonate, or a different dilute acid instead of dilute sulphuric acid. Recording your results 1 Write down what happened as you added the metal carbonate. 2 Write down the pH of the solutions at the start and at the end of these experiments. Considering your results/conclusions 3 a Did the reaction change the pH of the liquid? b What does this tell you about the reaction? Page 1 of 2 204 9Ec(3) Carbonates and acid (continued) 4 The experiment could also be carried out using this apparatus which allows the gas to be bubbled into limewater. If the limewater turns milky, which gas is released during the reaction? 5 Write a word equation for the reaction that has occurred between copper carbonate and dilute sulphuric acid. 6 Predict the names of the products formed for each of the reactions you carried out. I CAN... • carry out an investigation carefully • predict the products of a neutralisation reaction. 205 Page 2 of 2