Note 7
advertisement

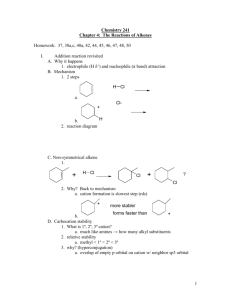
IB Chemistry Organic Chemistry Note 7 REACTION PATHWAYS (SL) Syllabus: 10.6 Textbook: This section deals with learning to deduce the reaction pathway for a two step process given the starting materials (reagents) and the required end product(s). You are also expected to be able to state the conditions needed for each step and write equations. Example: butanone. Describe a possible two step pathway for the conversion of but-2-ene to This can be done in two stages: 1. but-2-ene is heated with steam and a catalyst to form butan-2-ol, Note that this is an addition reaction ( reaction 7 below) 2. butan-2-ol, is a secondary alcohol and when it is oxidized by heating with acidified potassium dichromate(VI) will form butanone (a ketone). ( reaction 3 below) 9 4 5 1 12 6 2 10 7 8 11 3 The diagram above is taken from section 10.6 of the syllabus. The numbered reactions are described in more detail in each section below. Reaction 1. Converting alkane to halogenoalkanes General formula for an halogenoalkane: CnH2n+1X Reagents: chlorine, Cl2 or bromine, Br2 Conditions: in the presence of ultraviolet light CH4 + Br2 → CH3Br + HBr Further substitution occurs in succeeding reactions: CH3Br + Br2 → CH2Br2 + HBr CH2Br2 + Br2 → CHBr3 + HBr CHBr3 + Br2 → CBr4 + HBr Alkanes react with halogens by free radical substitution. In this reaction a hydrogen atom is removed from the alkane, then replaced by a halogen atom by reaction with a diatomic halogen molecule: Step 1. X2 → 2 X. (Initiation Step) Step 2. X. + R-H → R. + HX (1st Propagation Step) Step 3. R. + X2 → R-X + X. (2nd Propagation Step) Steps 2 and 3 keep repeating, each providing the reactive intermediate needed for the other step. This is called a radical chain reaction. Reactions 5 and 9: Further halogenation of halogenoalkanes to form dialogenoalkanes as well as tri and tetrahalogenoalkanes. These reactions are dealt with here because they have a similar mechanism to that descried above. Halogenoalkanes can be converted to dihalogenoalkanes by free radical substitution under uv light. This happens at room temperature and pressure and does not require a catalyst: e.g. CH3 CH2Br + Br2 → CH3 CHBr2 + HBr Dialogenoalkanes in turn can be converted to tri and tetrahalogenoalkanes. CH3 CHBr2+ Br2 → CH3 CBr3 + HBr CH3 CBr3 + HBr → CH2Br CBr3 + HBr Other position isomers are possible as the products. Reaction 2: Conversion of halogenoalkanes to form alcohol Usually aqueous NaOH is used to hydrolyze a Halogenoalkane. However some halogenoalkanes are reactive enough to hydrolyze in water. This is an example of a nucleophilic substitution reaction in which the OH group substitutes for the halogen in the halogenoalkane. Examples include: 1. C3H7Cl + OH- C3H8O + Cl2. CH3CH2Br + NaOH CH3CH2OH + NaBr Reagents: aqueous sodium hydroxide (usually with a little ethanol added to aid solution). Conditions: boil under reflux. The exact mechanism depends on the order of the carbon carrying the halogen and may be SN1 for tertiary halogenoalkanes or SN2 for primary halogenoalkanes. S N 1 mechanism R-X + OH ROH + X 2 step reaction (step 1 is rate determining) Reaction occurs with tertiary halogenoalkanes Step by Step: the C-Cl bond breaks heterolytically. (This is the weakest bond in the molecule) hydroxide ion acts as an electron donor and rapidly bonds with the positively charged carbon, forming a C-OH bond. S N 2 mechanism R-X + OH ROH + X 1 step reaction Mostly occurs with primary halogenoalkanes Step by Step: 1.) C-Cl bond is polar due to the electronegative nature of Cl 2.) The OH ion acts as a nucleophile and is attracted to the positively charged Carbon 3.) When the OH bonds to the Carbon the Cl is released simultaneously with the bonding pair (accounting for the negative charge ultimately) Reaction 3. Converting secondary alcohols to ketones Reagents: Secondary alcohol, Potassium dichromate (VI) solution and dilute sulphuric acid. Conditions: Heat under reflux (Reflux: a technique involving the condensation of gases and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to apply energy to reactions over a long period of time.) Equation: CH3CH(OH)CH3 + [O] → CH3COCH3 + H2O Example: [O] (Butan-2-ol) Ketone (Butanone) Reaction 4. Converting alkenes to alkanes This is an example of a hydrogenation reaction ( an addition reaction). With an addition of hydrogen an alkene is transformed into an alkane. The double bond is broken. Although this might seem like a “pointless” process this reaction is commonly used in food industry for example in making margarine from unsaturated plant oils using a nickel catalyst. Unsaturated fatty acids (alkene) may be converted to saturated (alkane) fatty acids by the relatively simple hydrogenation reaction. Reagent Alkene H2 gas Catalyst Conditions o Temperature: about 150°C o Pressure: relatively high o Catalyst: metal, e.g. ones with platinum, nickel, rhodium, or palladium bases (for lab purposes, an alloy of aluminium and nickel is used) Examples: Ethene reacts with hydrogen in the presence of a finely divided nickel catalyst at a temperature of about 150°C. Ethane is produced. Oil is heated with catalyst (Ni), heated to the desired temperature (140-225°C). Temperature: Hot temperatures (the more carbon-carbon double bonds the lower the melting point) Catalyst: Metal (usually heterogenous) catalyst- without catalysts the reaction would require much higher temperatures and pressures Platinum group metals, particularly platinum, palladium, rhodium, and ruthenium, form highly active catalysts, which operate at lower temperatures and lower pressures of H2 Non-precious metal like Nickel have been developed as economic alternatives, but are slower and require higher temperatures Pressure: (Concentration of H2) High pressure Reaction H2C=CH2 + H2 (g) alkene (ethene) + hydrogen gas CH3-CH3 alkane (ethane) The effects of Hydrogenation Alkene Alkane Unsaturated Saturated Liquid Solid Cis Trans Reaction 6. Conversion of alkenes to halogenoalkenes Reagents: hydrogen halide , symmetrical alkene Conditions: gas phase or in an inert solvent such as CCl4 at room temperature Equations: Symmetrical alkenes react with a hydrogen halide (HX) to form an alkyl halide. The double bond of the alkene is replaced by two new bonds, one to the halogen and one to the hydrogen atom of the hydrogen halide. For example, but-2-ene reacts with hydrogen bromide as follows: CH3CH=CH2CH3 + HBr → CH3CH(Br)CH3 Reaction 7. Converting Alkenes to alcohols Water only adds to alkenes in the presence of an acid catalyst. The result of the hydration is an alcohol. Key Concepts: Hydration is an addition reaction in which water adds across the double bond of the aslkene. This method is used to produce industrial ethanol from ethane. In industry direct hydration of ethene or other alkenes from cracking of fractions of distilled crude oil is used. This is carried out using phosphoric acid as the catalyst under high temperature and pressure. General Equation: Alkene + Water H+ Alcohol Reaction 8: Conversion of alcohol to aldehyde A primary alcohol can be oxidized to produce an aldehyde under the following conditions: oxidizing agents: H+(aq) / Cr2O72-(aq) 293-353 K atmospheric pressure Reaction 10. Converting alkenes to polyalkenes Alkenes can be bonded using a high pressure and a suitable catalyst. This process is known as addition polmerisation. The product of this addition process is a very long hydrocarbon chain (only hydrogen and carbon), which is a polymer. Since a polymer is a product of an addition reaction it is called an addition polymer and is known as a polyalkene. The optimal conditions for the polymerisation of alkenes can be deduced from Le Chatelier's principle: The reaction involves breaking double bonds, and since single bonds are made the reaction is exothermic. The reaction involves a reducing the total number of moles of the reactants. Since the reaction is exothermic, the greatest yield is obtained at low temperatures. In addition, to reduce the number of gas moles a high pressure is used. Reaction 11: Conversion of aldehyde to carboxylic Acid Aldehydes, RCHO, can be oxidized to carboxylic acids, RCO2H, in aqueous conditions. The reaction requires a mild oxidizing agent such as aqueous Cr (VI), Na2CrO4, or K2Cr2O7 and proceeds at normal atmospheric pressure and a warm temperature. Reacion 12 Conversion of alkenes into dihalogenoalkenes c) Alkene dihalogenoalkane Reagent: X2 in water or in an organic solvent Conditions: room T Equation: