Supplementary materials Supporting materials Analysis of SSAO
advertisement

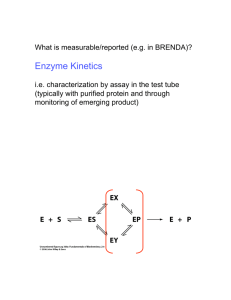
Supplementary materials Supporting materials Analysis of SSAO/VAP-1 kinetic data is available as supporting material. Analysis of SSAO/VAP-1 kinetic data. The following reaction scheme 1 developed by Holt et al. (2008) was considered. E is the free enzyme, and ES is used to refer both to oxidized form enzyme with bound substrate and to reduced form of enzyme (reaction intermediate after release of aldehyde product). ESI and ESS are species resulting after binding of inhibitor or substrate, respectively, to ES. Ki is dissociation constant for ESS complex, and KIE and KIES dissociation constants for free enzyme-inhibitor complex (EI) and (enzyme-substrate)-inhibitor complex (IES), respectively. Parameters “f and α” are factors indicating degree to which catalytic constant decreases if ESI and the ESS complexes have residual activity, respectively. In absence of inhibitor, fitting these experimental data (initial rate of reactions versus substrate concentration) to eq. 2 of Holt et al. (2008), yielded a dissociation constant (Ki)= 25 ±5 mM and α=0.3. Thus, it is deduced that, in our experimental conditions ([benzylamine]<10 mM), term “α×[S]/KIS” (in eq. 2 of Holt et al. 2008) is negligible and the following simplified equation was obtained (eq. 1 in text): v Vmax ,app [ S ] 2 S K m app S K I K i i K IES (S1) The apparent kinetic parameters in this equation are: Vmax 0 (1 f [ I ] / K IES ) (S2) (1 [ I ] / K IES ) Vmax app and K m app K m 0 1 [ I ] / K IE (S3) (1 [ I ] / K IES ) The apparent catalytic efficient is: Vmax app K m, app Vmax 0 (1 f [ I ] / K IES ) (S4) Km0 (1 [ I ] / K IE ) To understand mode of inhibition of ELP 12 and Bis-Bza-Diado to SSAO/VAP-1, (1/Vmax)app and (Km/Vmax)app values were calculated, applying eq.1, and plotted against inhibitor concentration (in 20-500 μM range). Fitting eqs.S2 and S4 to apparent kinetic parameters vs inhibitor concentration yielded a bad fit and “f/KIES<<0” was found. These equations are non-linear functions, whereas our kinetic data clearly show linear dependence of (1/Vmax)app and (Km/Vmax)app on [I] (see Fig. 3). Consequently, in explored inhibitor concentration range, eqs.S2 and S4 may be simplified to yield the following “classical equations” for simple, mixed, non-competitive inhibition (Leidler and Bunting 1973): 1 Vmax app K m app Vmax app Vmax 0 [I ] (S5) 1 K IES K m0 Vmax 0 [I ] (S6) 1 K IE 1 S5 and S6 correspond to equations 2 and 4 in text. Fitting these equations to given kinetic data yielded a good fit (see Fig. 3). Accordingly, scheme 1 may be simplified to: References Leidler K, Bunting P (1973) The Chemical Kinetics of Enzyme Action”. (2nd edn.) Clarendon Press, Oxford, pp. 89138 Holt A, Smith DJ, Cendron L, Zanotti G, Rigo A, Di Paolo ML (2008) Multiple binding sites for substrates and modulators of semicarbazide-sensitive amine oxidases: kinetic consequences. Mol Pharm 73:525-538 doi: 10.1124/mol.107.040964