biological toxins
advertisement

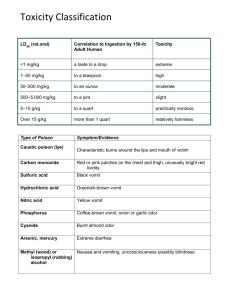
BIOSAFETY REGISTRATION FORM FOR BIOLOGICAL TOXINS Biological Toxin: Lab Principal Investigator: Department: Building & Room: Prepared By: PI Approval Signature: Initial Approval Date: Annual Registration Form Reviews Reviewer Annual Review Date Page 1 of 12 When should this Biosafety Registration Form be completed? Biosafety level 2 (BSL-2) laboratories are required to prepare a laboratory-specific biosafety manual. A Biosafety Registration Form should be completed for each procedure involving work with toxins of biological origin. Each laboratory should maintain a copy of their registration forms with their hard copy of the University Biosafety Manual. The addition of these registration forms to the general University Biosafety Manual creates a laboratoryspecific manual. What are the benefits of completing a Biosafety Registration Form? 1. They are reviewed by the Biosafety Committee (IBC), which is composed of peer researchers with expertise to ensure biosafety considerations have been addressed. 2. They serve as written reminders to the staff of the correct way of conducting a procedure. 3. They should be used as training tools for all new staff. A record should be maintained to indicate which registration forms have been read and understood by the employee. This same record can be used to document the date an employee is competent to perform the procedure as outlined in the registration form. 4. Completing a Biosafety Registration Form forces the author to think through the procedure step by step and to modify those procedures if necessary. 5. The use of written Registration Forms allows your lab to ensure each researcher utilizes the appropriate work practices, safety equipment, and laboratory facilities to reduce the risk of exposures, while also maintaining compliance with applicable university policies, and external regulations and guidelines. What are the primary considerations when completing a Biosafety Registration Form? The registration form responses should be succinct to assure that they will be read and frequently referenced. The individual(s) who actually conduct the procedure on a daily basis should be the ones to complete the registration form since they are most familiar with the procedure. The PI should review and approve the registration form before it is implemented and the procedures are performed by other researchers in the lab. How should the lab distribute and archive a Biosafety Registration Form? A copy of the registration form should be available at all work stations where the biological toxin is used, not in a file cabinet or on a shelf in the supervisor's office. The registration forms are working documents and must be available to your staff for reference and use. Each person working with the biological toxin should be familiar with the applicable biosafety registration form. A copy of each form should also be maintained in the laboratory with the other biosafety resources. Page 2 of 12 BIOSAFETY REGISTRATION FORM FOR WORK INVOLVING TOXINS OF BIOLOGICAL ORIGIN Please answer the following questions related to your research involving work with toxins of biological origin. Special considerations have been added to several of the questions to provide additional guidance on recommended biosafety practices. 1. Experimental Procedures: Provide a summary of the nature and purpose of the research involving biological toxins. Describe the experimental procedures and techniques to be utilized in the project. Include a risk assessment that considers factors such as the toxin under study, the physical state of the toxin (liquid solution or powder form), the total amount of toxin used relative to the estimated human lethal dose, the volume of the material manipulated, the methodology, and any human or equipment performance limitations. Will experiments involving biological toxins also involve the use of nanomaterials? YES NO If YES, indicate the type of nanomaterials used for these experiments (e.g. carbon nanotubes, gold nanoparticles). 1. Biological Toxin Used: List the biological toxin used in the procedure. 2. HHS & USDA Select Agents and Toxins? a) Is the toxin included on the HHS & USDA Select Agents and Toxins list? This list can be viewed online at http://origin.cdc.gov/od/sap/docs/salist.pdf YES Page 3 of 12 NO b) If YES, What is the maximum toxin amount excluded from regulation? Toxin excluded amounts online at http://origin.cdc.gov/od/sap/sap/toxinamt.htm 3. Training: a) List any general training requirements for personnel working with the toxin (i.e. Laboratory Safety Training, Biosafety Training, Bloodborne Pathogens Training). b) Describe any toxin-specific training provided by the laboratory supervisor to ensure that lab personnel are adequately trained regarding their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures. Special Considerations: This may include how to handle transfers of liquids containing toxin, where to place waste solutions and contaminated materials or equipment, and how to decontaminate work areas after routine operations, as well as after accidental spills. 4. Toxin Ordering: a) Where will the toxin be ordered from? (e.g. commercial source). b) List the maximum toxin amount that will be ordered at one time, and the anticipated frequency of ordering. 5. Toxin Storage: a) List the maximum total amount of toxin that would be stored in the lab at any time. b) List the building, room number, and appliance where the toxin will be stored. c) Describe how the toxin containers in the lab are sealed, labeled, and secured to ensure restricted access. Page 4 of 12 6. Toxin Transport: a) Is the biological toxin transported between labs and/or buildings? YES NO If YES, describe the procedures for transporting samples (i.e. durable and leak-proof secondary containers). 7. Inventory Control: a) Describe the inventory control system in place to account for toxin use and disposition. This inventory control system should include logs for the toxin amounts received and date, amounts used and date, amounts disposed and date. 8. Risks/Hazards: a) Identify “critical steps” in the procedure that may create a risk of exposure during sample preparation and/or experimental manipulations (i.e. reconstitution of toxin powder into solution). b) Describe the potential routes of exposure that may be encountered. These may include accidental exposure by direct contamination of mucous membranes (i.e. eyes, nose, or mouth), inhalation of aerosols, and needle-stick or sharps cut causing the skin to break. 9. Hazard Communication: a) Describe any hazard communication signage that will be posted when the toxin is in use. For instance, the entrance door to the lab room may have posted signage indicating: “Toxins in Use – Authorized Personnel Only.” Page 5 of 12 10. Engineering Controls: a) Describe the engineering controls that will be utilized. Special Considerations: This may include all work being performed in a certified biological safety cabinet or chemical fume hood. Engineering controls should be selected according to the risk assessment for the specific toxin operation. b) Describe if the work will involve volatile chemicals or radionucleotides combined with toxin solutions. 11. Personal Protective Equipment (PPE): a) Describe any PPE that must be worn when performing the procedure. Special Considerations: This may include lab coats and disposable gloves. When working with toxins that pose direct percutaneous hazards, special care must be taken to select gloves that are impervious to the toxin and the diluents or solvents employed. b) Describe PPE for liquid transfers. Special Considerations: When conducting liquid transfers and other operations that pose a potential splash or droplet hazard in an open-fronted hood or BSC, safety glasses and disposable facemask, or a face shield, should be worn. c) Describe any considerations for removal or decontamination of reusable PPE. Protective clothing should be removed before leaving for non-laboratory areas. 12. Decontamination: a) Describe the decontamination of work surfaces. For instance, all work surfaces will be decontaminated after completion of work and after any spill. b) Describe the decontamination of primary containers. Page 6 of 12 Special Considerations: The toxin should be removed from the hood or BSC only after the exterior of the closed primary container has been decontaminated and placed in a clean secondary container. c) Describe the decontamination of lab equipment. Special Considerations: The interior of the hood or BSC should be decontaminated periodically, for example, at the end of a series of related experiments. 13. Toxin Waste Disposal: a) Describe how each type of toxin waste generated during the procedure will be properly disposed (i.e. autoclave, sharps container, chemical disinfection). Please evaluate the following considerations when determining the appropriate disposal method: Toxin stability varies considerably outside of physiological conditions depending upon the temperature, pH, ionic strength, and other characteristics. Inactivation is not always a linear function of heating time, and some protein toxins possess a capacity to re-fold, and partially reverse inactivation caused by heating. In addition, the conditions for denaturizing toxins in aqueous solutions are not necessarily applicable for inactivating dry, powdered toxin preparations. Many toxins are susceptible to inactivation with dilute sodium hypochlorite (NaOCl) bleach solutions at concentrations of 0.1-0.5%. Use freshly prepared bleach solutions for decontamination; undiluted, commercially available bleach solutions typically contain 3-6% NaOCl. Depending upon the toxin, contaminated materials and toxin waste solutions can be inactivated by incineration or extensive autoclaving, or by soaking in suitable decontamination solutions. All disposable material used for toxin work should be placed in secondary containers, autoclaved and disposed of as hazardous waste. Contaminated or potentially contaminated protective clothing and equipment should be decontaminated using suitable chemical methods or autoclaving before removal from the laboratory for disposal, cleaning or repair. If decontamination is impracticable, materials should be disposed of as hazardous waste. Materials to be decontaminated outside the immediate lab must be placed in a durable, leak-proof container and secured for transport. 14. Sharps & Mechanical Injuries: a) Describe each type of sharps device utilized during the procedure. These may include Page 7 of 12 needles, scalpels or glass pipettes. b) Describe how each type of sharps device will be properly disposed. Special Considerations: Accidental needle-sticks or mechanical injury from “sharps” pose a well-known risk to lab workers, and the consequences may be catastrophic for operations involving toxins in amounts that exceed a human lethal dose. Discarded needles/syringes and other sharps should be placed directly into properly labeled, puncture-resistant sharps containers, and decontaminated as soon as practical. Needles should never be bent, broken, recapped, removed from disposable syringes, or otherwise manipulated by hand before disposal. c) Describe how glassware will be replaced with plastic for handling toxin solutions wherever practical to minimize the risk of cuts or abrasions from contaminated surfaces. Special Considerations: Thin-walled glass equipment should be completely avoided. Glass Pasteur pipettes are particularly dangerous for transferring toxin solutions and should be replaced with disposable plastic pipettes. Glass chromatography columns under pressure must be enclosed within a plastic water jacket or other secondary container. 15. Toxin Aerosols: a) Describe how the lab has evaluated and modified experimental procedures to eliminate the possibility of inadvertent generation of toxin aerosols. Special Considerations: Pressurized tubes or other containers holding toxins should be opened in a BSC, chemical fume hood, or other ventilated enclosure. Operations that expose toxin solutions to vacuum or pressure, for example sterilization of toxin solutions by membrane filtration, should always be handled in this manner, and the operator should also use appropriate respiratory protection. If vacuum lines are used with the toxin, they should be protected with a HEPA filter to prevent toxin entry into the line. b) Describe any additional precautions for minimizing the generation of aerosols when centrifuging samples. Special Considerations: Centrifugation of materials potentially containing toxins should only be performed using sealed, thick-walled tubes in safety centrifuge cups or sealed rotors. After centrifugation, the entire rotor assembly should be taken from the centrifuge to a BSC to open it and remove its tubes. Page 8 of 12 16. Toxin Spill Clean-up: a) Describe the procedures that will be followed in the event of a spill involving the toxin. The procedure should include the materials necessary for clean-up, how to avoid splashes or aerosols, and the type of disinfectant that will be used. Special Considerations: Avoid splashes or generating aerosols during cleanup by covering the spill with paper towels or other disposable, absorbent material. Apply an appropriate decontamination solution to the spill, beginning at the perimeter and working towards the center, and allow sufficient contact time to completely inactivate the toxin. 17. Injury/Exposure Response: a) Describe the response procedures that will be followed in the event of a laboratoryacquired injury/exposure. Include any initial treatment (i.e. washing exposed area), where to seek medical treatment, and any necessary emergency contact numbers. 18. Toxin Administration in Animals: a) Will the biological toxin be administered into live animals? YES NO If YES, continue answering questions 19. b - d b) Describe the route of administration and animal species receiving the toxin. c) Describe the concentration and volume of the toxin administered. d) Describe the type of training provided to workers administering toxin to animals. Special Considerations: Only workers trained and experienced in handling animals should be permitted to conduct operations involving injection of toxin solutions using hollow-bore needles. 19. Laboratory Facilities: Page 9 of 12 a) Check each of the following that are readily available in the laboratory where the biological toxin is used: Eyewash station Door(s) with locks Sink for hand washing Furniture that can be easily cleaned/decontaminated Biological safety cabinet(s) with updated annual certification Chemical Fume Hood(s) with updated annual certification ADDITIONAL PRECAUTIONS & REQUIRED APPROVALS Experiments should be planned to eliminate or minimize work with dry toxin (e.g. freeze-dried preparations). Unavoidable operations with dry toxin should only be undertaken with appropriate respiratory protection and engineering controls. Dry toxin can be manipulated using a Class III BSC, or with the use of secondary containment such as a disposable glove bag or glove box within a hood or Class II BSC. “Static-free” disposable gloves should be worn when working with dry forms of toxins that are subject to spread by electrostatic dispersal. In specialized laboratories, the intentional, controlled generation of aerosols from toxin solutions may be undertaken to test antidotes or vaccines in experimental animals. These are extremely hazardous operations that should only be conducted after extensive validation of equipment and personnel. Also, some toxins are included on the HHS/USDA Select Agents and Toxins list, although toxins included on this list may be excluded from the regulations if used in amounts below the regulated limits. Any planned experiments involving the controlled generation of toxin aerosols, or use of a toxin included on the HHS/USDA Select Agents and Toxins list must receive prior approval from the USC Department of Environmental Health and Safety (EH&S) and Institutional Biosafety Committee (IBC). For high-risk operations involving dry forms of toxins, intentional aerosol formation, or the use of hollow-bore needles in conjunction with amounts of toxin estimated to be lethal for humans, consideration should be given to requiring the presence of at least two knowledgeable individuals at all times in the laboratory. SELECT AGENT TOXINS Due diligence should be taken in shipment or storage of any amount of toxin. There are specific regulatory requirements for working with toxins designated as a “Select Agent” by the DHHS and/or the USDA. Select Agents require registration with CDC and/or USDA for possession, use, storage and/or transfer. Importation of this agent may require CDC and/or USDA importation permits. Domestic transport of the agent may require a permit from USDA/APHIS. A Department of Commerce (DoC) permit may be required for the export of the agent to another country. CERTIFICATION OF UNDERSTANDING: Page 10 of 12 All laboratory personnel working with the biological toxin described in this Biosafety Registration Form will be required to sign the following. I have fully read all sections of this Biosafety Registration Form for work involving biological toxins. I understand all the information included in this form and agree to follow the described procedures. I understand that I may ask questions or request additional information from my laboratory PI/supervisor if I have concerns regarding the duties I will be performing, or the necessary precautions to prevent exposures. I also understand that I may contact the Department of Environmental Health and Safety to discuss any concerns with the implementation of this registration form. Name Signature Date PI Initials ________________________________________________ IBC Use Only IBC Meeting Review Date: All revisions recommended by the Biosafety Officer have been appropriately addressed? YES NO All revisions recommended at the IBC meeting have been appropriately addressed? YES NO Principal Investigator must maintain a signed copy of their registration form that indicates all revisions recommended by the BSO and IBC were appropriately addressed Page 11 of 12 Page 12 of 12