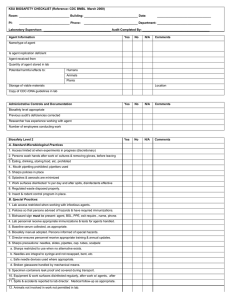
Biosafety Registration Amendment Form
advertisement
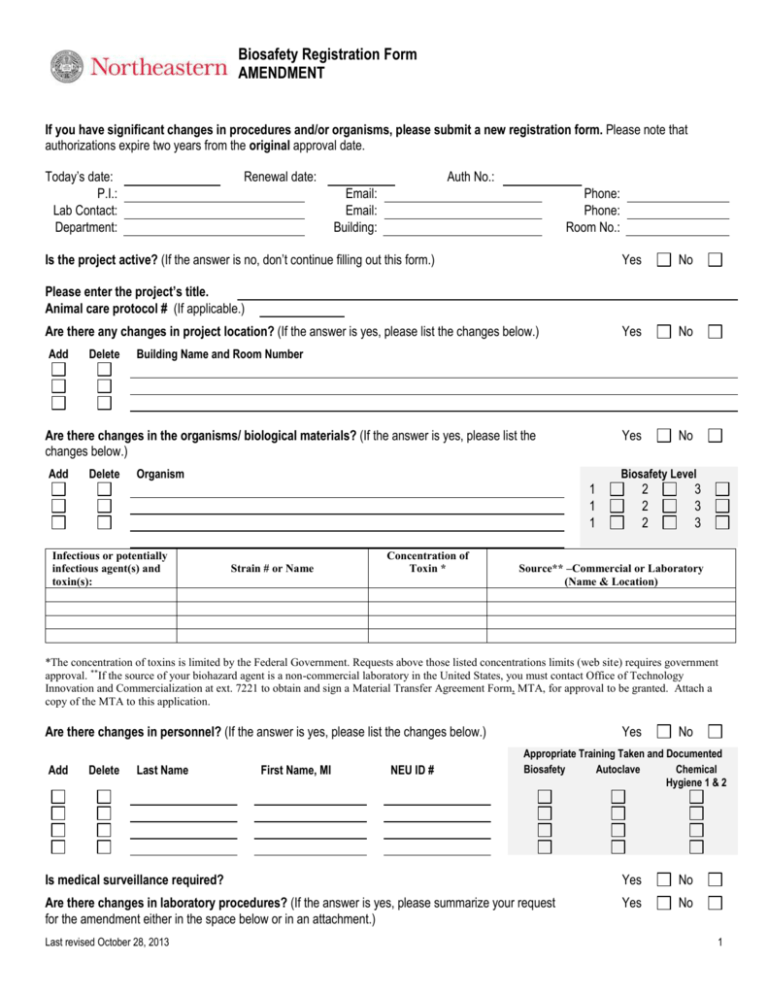
Biosafety Registration Form AMENDMENT If you have significant changes in procedures and/or organisms, please submit a new registration form. Please note that authorizations expire two years from the original approval date. Today’s date: P.I.: Lab Contact: Department: Renewal date: Auth No.: Email: Email: Building: Phone: Phone: Room No.: Is the project active? (If the answer is no, don’t continue filling out this form.) Yes No Yes No Yes No Please enter the project’s title. Animal care protocol # (If applicable.) Are there any changes in project location? (If the answer is yes, please list the changes below.) Add Delete Building Name and Room Number Are there changes in the organisms/ biological materials? (If the answer is yes, please list the changes below.) Add Delete Organism Biosafety Level 1 1 1 Infectious or potentially infectious agent(s) and toxin(s): Strain # or Name Concentration of Toxin * 2 2 2 3 3 3 Source** –Commercial or Laboratory (Name & Location) *The concentration of toxins is limited by the Federal Government. Requests above those listed concentrations limits (web site) requires government approval. **If the source of your biohazard agent is a non-commercial laboratory in the United States, you must contact Office of Technology Innovation and Commercialization at ext. 7221 to obtain and sign a Material Transfer Agreement Form, MTA, for approval to be granted. Attach a copy of the MTA to this application. Are there changes in personnel? (If the answer is yes, please list the changes below.) Add Delete Last Name First Name, MI NEU ID # Yes No Appropriate Training Taken and Documented Biosafety Autoclave Chemical Hygiene 1 & 2 Is medical surveillance required? Yes No Are there changes in laboratory procedures? (If the answer is yes, please summarize your request for the amendment either in the space below or in an attachment.) Yes No Last revised October 28, 2013 1 Biosafety Registration Form AMENDMENT PI’s signature: Last revised October 28, 2013 Date: 2