Clonogenic assays
advertisement

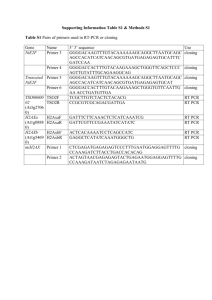
Supplementary Materials and Methods Transposon insertion site analysis. Three weeks post-transfection, G418-resistant HeLa colonies were picked and transferred to 96-well plates. When the cells reached confluency they were washed in PBS and frozen. To isolate genomic DNA, cells were lysed in 40 l Lysis buffer per well [10 mM Tris (Roth)/HCl, pH 7.5; 10 mM EDTA (Merck); 10 mM NaCl (Merck); 0.5% SDS (Roth); freshly add 1 mg/ml proteinase K (Merck)] for 2 hours at 60°C. After centrifugation (Eppendorf, model 5810 R) of the 96-well plates for 2 minutes at 4000 rpm and 20°C, 80 l per well of cold 75 mM NaCl/ethanol solution were added and plates were incubated for 1 hour at room temperature. Plates were washed three times with 150 l 70% ethanol for 2 minutes at 4000 rpm, and dried for 10 minutes using a vacuum centrifuge (Eppendorf concentrator 5301). Finally, 40 l 5mM Tris/HCl, pH 8.0 per well were added and plates were incubated overnight at room temperature. The splinkerette adaptor was prepared as follows (for one well): 0.15 l 10 M primer 40_SpAa.1, 0.15 l 10 M primer 41_SpBb.1, 0.05 l 10x SuRE buffer M (Roche) and 0.65 l bidestilled water were mixed, heated to 97 °C for 4 minutes and slowly cooled down to room temperature. To prepare the plates for sequencing, a PCR protocol was performed in 10 l reactions per well including 1 l second step PCR product, 1 l Big Dye Terminator mix (MPI for Molecular Genetics, Sequencing Group, Berlin), 1 l 10 µM primer, and 7 l PCRgrade water. The temperature profile was 1 minute at 96 °C, 25 cycles at 96 °C for 10 seconds, 54 °C for 5 seconds, and 60 °C for 4 minutes. Products were precipitated by adding 22 l per well of a 3 mol/L Sodium acetate/96% ethanol solution. 96-well plates were incubated for 10 minutes at room temperature on a shaker and afterwards centrifuged at 4000 rpm for 50 minutes. The supernatant was decanted and the plates were washed twice with 100 l per well 70 % ethanol at 4000 rpm for 15 minutes. Western blotting. 48 hours post transfection, HeLa cells were lysed in RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) supplemented with protease inhibitor cocktail (Complete Mini, Roche, Basel, Switzerland). Total protein was quantified using BCA Protein Assay Kit (Pierce, Rockford, IL). Proteins (10 g per lane) were loaded onto 10% polyacrylamide gel and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Gels were transferred to nitrocellulose membrane (Hybond–C Extra, Amersham Bioscience) and immunoblotting was performed according to standard procedures. Proteins were detected with rat monoclonal anti-HA antibody (Roche, Basel, Switzerland) dilution 1:2000, or mouse monoclonal anti-actin antibody (Dianova, Hamburg, Germany) dilution 1:800, and chemiluminescence using ECL Plus Western Blotting Detection Kit (Amersham Bioscience). Clonogenic assays. Colony assays were performed by adding 50 l of Stemline medium (Sigma-Aldrich, USA) supplemented with 100 ng/ml SCF, 20 ng/ml IL-6, 100 ng/ml IL-3, 20 ng/ml Flt3-L and 100 ng/ml TPO to the 100 l of electroporated CD34+ cell suspension. Granulocyte/monocyte/macrophage clonogenic assays (CFU-GM) were performed by adding 30 l of the final cell suspension to 270 l of granulocyte/monocyte/macrophage differentiation medium corresponding to semi-solid Methocult GF H4534 (Stemcell Technologies, Vancouver Canada) composed of 1% methylcellulose (4000 cps), 30% fetal bovine serum, 1% bovine serum albumin, 10-4 M 2-mercaptoethanol, 2 mM L-glutamine, 50 ng/ml rhSCF, 10 ng/ml rhGM-CSF, 10 ng/ml rhIL-3 in Iscove’s Modified Dulbecco’s Medium (MDM). The cell suspensions were seeded over 3 wells in a 24-well plate at a concentration of 5x104 cells per well. At day 14, GFP+ and total CFU-GM colonies were counted using a fluorescence inverted microscope (Olympus, Japan). GFP expression and scoring of CFU-Mk colonies was monitored using the Olympus fluorescence microscope. In addition confocal microscopy was conducted at 488 nm with an Axiovert 100M, LSM510, Zeiss using the AxioPlan 2 LSM 510 version 2.8 software. Erythroid clonogenic assays (CFU-E) were performed by adding 30 l of the final cell suspension to 270 l of erythoid differentiation medium corresponding to semi-solid Methocult SFBIT H4436 (Stemcell Technologies, Vancouver, Canada) composed of methylcellulose, fetal bovine serum, bovine serum albumin, 2-mercaptoethanol, L-glutamine, rhSCF, rhGM-CSF, rhIL-3, rhIL-6, rhG-CSF, rh Epo in Iscove’s MDM. The cell suspensions were seeded over 3 wells in a 24-well plate, hence containing 5x104 cells per well. At day 7, colonies were counted which typically contained about 70% glycophorin A+ cells, a characteristic marker of erythroid cells. GFP+ CFU-E colonies were monitored as mentioned above. Cytologic analysis was performed by Giemsa staining. Oligonucleotides Primer name Length (bp) SB/XhoI/Kozak 41 Cloning of pCSB100XNpA SB/NotI 46 Cloning of pCSB100XNpA Tol2/XhoI/Kozak 39 Cloning of pCTol2NpA Tol2/NotI 40 Cloning of pCTol2NpA mPB/XhoI/Kozak 41 Cloning of pCmPBNpA mPB/NotI 40 Cloning of pCmPBNpA Usage Sequence (5’ – 3’) TACCACTCGAGCCACCA TGGGAAAATCAAAAGAA ATCAGCC AGCAACTAGCGGCCGCC TAGTATTTGGTAGCATTG CCTTTAAATTG TACCACTCGAGCCACCA TGGAGGAAGTATGTGAT TCATC AGCAACTAGCGGCCGCC TACTCAAAGTTGTAAAA CCTCAG TACCACTCGAGCCACCA TGGGCAGCAGCCTGGAC GACGAGC AGCAACTAGCGGCCGCT CAGAAACAGCTCTGGCA CATGTC Tm (°C) 120 134 116 120 136 126 iPB/XhoI/Kozak 41 Cloning of pCiPBNpA iPB/NotI 40 Cloning of pCiPBNpA Cloning of transposase constructs Cloning of transposase constructs poly-A/NotI 36 poly-A/SacI 36 SB-HA/NotI 100 Cloning of pCSB100XHApA Tol2-HA/NotI 94 Cloning of pCTol2HApA mPB-HA/NotI 101 Cloning of pCmPBHApA SB 34 Cloning of pUC19SBneo Tol2L 40 Cloning of pUC19Tol2 neo Tol2R 41 Cloning of pUC19Tol2 neo PBL 32 PBR 28 40_SpAa.1 61 Splinkerette adaptor 48 Splinkerette adaptor with BstYI recognition sequence 41_SpBb.1 Cloning of pUC19PBneo Cloning of pUC19PBneo TACCACTCGAGCCACCA TGGGTAGTTCTTTAGAC GATGAGC AGCAACTAGCGGCCGCT CAGAAACAACTTTGGCA CATATC AGCAACTTGCGGCCGCG TTGTTAACTTGTTTATTG C AACGTACCGAGCTCGAT TTAACAAAAATT TAACGCG AGCAACTAGCGGCCGCT TAGGCATAATCCGGTAC ATCATAAGGGTAGGCAT AATCCGGTACATCATAA GGGTAGTATTTGGTAGC ATTGCCTTTAAATTG AGCAACTAGCGGCCGCT TAGGCATAATCCGGTAC ATCATAAGGGTAGGCAT AATCCGGTACATCATAA GGGTACTCAAAGTTGTA AAACCTCAG AGCAACTAGCGGCCGCT TAGGCATAATCCGGTAC ATCATAAGGGTAGGCAT AATCCGGTACATCATAA GGGTAGAAACAGCTCTG GCACATGTCGATGTTG TATCTTTACAGTTGAAGT CGGAAGTTTACATACA TATCTTTACAGAGGTGT AAAGTACTTGAGTAATT TTACTT TATCTTTACAGAGGTGT AAAAAGTACTCAAAAAT TTTACTC TTAACCCTAGAAAGATA ATCATATTGTGACGT TTAACCCTAGAAAGATA GTCTGCGTAAA CGAAGAGTAACCGTTGC TAGGAGAGACCGTGGCT GAATGAGACTGGTGTCG ACACTAGTGG GATCCCACTAGTGTCGA CACCAGTCTCTAATTTTT TTTTTCAAAAAAA 124 120 106 100 288 274 300 90 102 104 84 76 - - Splinkerette adaptor with NspI 52_SpNspI 48 recognition sequence First round adaptor 38_Sp0F.2 28 primer Second round 39_Sp1F.2 25 adaptor primer First nested primer 72 24 for 3' site of SB insertions First nested primer 73 27 for 5' site of SB insertions Second nested primer for 5' and 3' T-Bal 27 site of SB insertions and sequencing primer First nested primer 70 18 for 5' site of mPB insertions First nested primer 71 21 for 3' site of mPB insertions Second nested primer for 5' site of XLfor 25 mPB insertions and sequencing primer Second nested primer for 3' site of XLrev 22 mPB insertions and sequencing primer First nested primer 74 22 for 5' site of Tol2 insertions First nested primer 81 24 for 3' site of Tol2 insertions Second nested primer for 5' site of 61 25 Tol2 insertions and sequencing primer Second nested primer for 3' site of 75 24 Tol2 insertions and sequencing primer Tm, melting temperature of the primers in °C CATGCCACTAGTGTCGA CACCAGTCTCTAATTTTT TTTTTCAAAAAAA CGAAGAGTAACCGTTGC TAGGAGAGACC GTGGCTGAATGAGACTG GTGTCGAC - 64 63 CACCCTAACTGACACAC ATTCCAC 60 CTCATCAATGTATCTTAT CATGTCTGG 57 CTTGTGTCATGCACAAA GTAGATGTCC 60 CCGGGCTGCAGGAATTC G 60 GACAAGCACGCCTCACG GGAG 64 TACAGACCGATAAAACA CATGCGTC 58 CCAAGCGGCGACTGAGA TGTCC 63 CATGTCTGGATCGACTA CTAGG 58 GTATCTTATCATGTCTGG ATCGAC 56 CTCAAGTAAGATTCTAG CCAGATAC 56 GACTGTAAATAAAATTG TAAGGAG 51 Supplementary Figure Legends Supplementary Figure 1. Levels of SB100XHA, Tol2HA and mPBHA transposase expression in HeLa cells. (a) Schematic representation of HA-tagged transposase constructs. CAGGS, CAGGS promoter; pA, polyadenylation sequence; SB100X, hyperactive SB transposase; Tol2, Tol2 transposase; mPB, mouse codon-optimized PB transposase, HA, double HA-tag added to the C-terminus of each transposase. (b) Western blot analysis of SB100XHA, Tol2HA and mPBHA transposase expression. The transfected amounts of transposase expression plasmids correspond to those and higher than required for the peak activity of each system (i. e., ≥5 and 50 ng for SB100X, ≥125 and 250 ng for Tol2 and ≥50 and 250 ng for mPB). Supplementary Figure 2. Relative transposition efficiencies of SB100X, Tol2 and PB transposons in “low” and “high” transposon DNA conditions. Transposition activity was measured at the peak conditions for both the low (10 ng) (a) and the high (500 ng) (b) dosages of transfected transposon plasmids by determining the numbers of antibiotic-resistant colonies per transfected cell population multipled by the average numbers of transposon insertions per colony for each of the three transposon systems. The graphs show average numbers of transposon insertions per 100 transfected cells. Error bars represent standard error of the mean (SEM). Supplementary Figure 3. Persistence of transposition-mediated gene expression in human HeLa cells. Photographs of representative, exemplary cell colonies stably transfected with Venus-tagged transposon vectors are shown. (a) Uniformly expressing colony showing no signs of transgene silencing; (b) uniformly silenced colony that lost Venus expression; (c) mosaic colony showing sectors with cells that lost Venus expression; (d) uniformly silenced colony with attenuated Venus expression. Supplementary Figure 4. Sequence logo analysis of SB100X, Tol2 and PB integration sites. Web logo analyses and nucleotide probability plots of 46 SB100X (a), 113 Tol2 (b) and 46 PB (c) integration sites in HeLa cells. Supplementary Table 1. Distribution of SB, PB and Tol2 integration sites in vertebrate genomes. The table summarizes results produced by this study on the distribution of Tol2 transposon insertions in human HeLa cells in the context of transposon mapping data obtained by others. Values typed in bold are significantly different from random datasets. b Insertions mapped in this study were obtained with a Tol2 transposon-based enhancer trap cassette remobilized in two transgenic donor lines. cValues for insertions only upstream of genes were determined. ND – not determined. References: 1. Yant SR, Wu X, Huang Y, Garrison B, Burgess SM, Kay MA (2005). High-resolution genome-wide mapping of transposon integration in mammals. Mol Cell Biol; 25: 2085-2094.; 2. Wilson MH, Coates CJ, George AL, Jr. (2007). PiggyBac transposon-mediated gene transfer in human cells. Mol Ther; 15: 139-145.; 3. Galvan DL, Nakazawa Y, Kaja A, Kettlun C, Cooper LJ, Rooney CM, et al. (2009). Genome-wide mapping of piggyBac transposon integrations in primary human T cells. J Immunother. 32: 837-844.; 4. Kondrychyn I, Garcia-Lecea M, Emelyanov A, Parinov S, Korzh V (2009). Genome-wide analysis of Tol2 transposon reintegration in zebrafish. BMC Genomics; 10: 418.