Supplementary Information (doc 61K)
advertisement

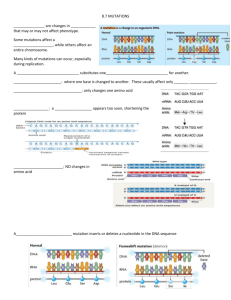
Supplementary Materials and Methods Histology and immunohistochemistry Sections from formalin-fixed, paraffin-embedded specimens were stained with hematoxylin/eosin. The antibodies used for immunohistochemistry were: CD117 and S100 (rabbit polyclonal), and vimentin (mouse monoclonal) (DAKO, Glostrup, Denmark, 1:400, 1:800 and 1:200, respectively), DOG1 (Spring Bioscience, Pleasanton, CA, rabbit polyclonal, 1:100), CD34 (Novocastra, Newcastle, UK, 1:50) and PDGFRA (sc-338) (Santa Cruz, Santa Cruz, CA, rabbit polyclonal, 1:400). Antigen retrieval was performed for DOG1, S100, vimentin and PDGFRA (10 minutes in 0.01 M citrate buffer, pH 6, 750 W microwave). Specific pre-immune sera or isotype-specific unrelated primary antibodies were used for the controls. Hydrogen peroxide, serum biotinylated immunoglobulins, and avidin-biotin complexes were used following the manufacturer's instructions; after color induction with diaminobenzidine solution (Dakopatts, Glostrup, Denmark), slides were counterstained with hematoxylin. Sequence analysis in KIT and PDGFRA genes Informed written consent was obtained from all tested individuals. DNA was obtained from paraffinembedded tissues or buccal swab samples (the latter performed in all patients tested except patient II-2 and II-3, where formalin-fixed, paraffine embedded tissues were available first). Slides were cut from paraffin-embedded tissues of patients II-2, II-3, III-4 and III-5, treated twice with xylene and washed with ethanol; pathologic areas (nearly 100% disease-specific tissue) were macro-dissected on slides; mutational analysis was also performed in normal tissue (gastrointestinal wall layers, or lymph nodes). DNA was extracted using the QIAamp tissue mini kit (Qiagen, Hilden, Germany). KIT (exons 9, 11 13, and 17) and PDGFRA (exons 12, 14, and 18) genes were amplified using the same primers and PCR conditions described elsewhere(1-3). Briefly, DNA (100-200 ng) was amplified in a mixture containing 1xPCR buffer [20 mM TRIS (pH 8.3), 50 mMKCl, 1.5 mM MgCl2], dNTPs (200 mM each), primers (20 pM each), and 0.5 U Taq polymerase platinum (Invitrogen, Milan, Italy) in a 25 l final volume. PCR conditions were: 8-min initial denaturation at 95°C, then 35 cycles at 95°C for 40s, 55°C for 40s, and 72°C for 40s. After visualization onto agarose gel, PCR products were treated with ExoSAP-IT (USB Corp, Cleveland, OH) following the manufacturer's protocol, amplified with BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems) using forward and reverse primers, and sequenced with an ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems). Water was the negative control. Bioinformatics analysis The P653L PDGFRA missense mutation was predicted with 4 different computational tools: “SIFT”, a predictor based on the degree of conservation of amino-acid residues in sequence alignments derived from closely related sequences(4); “PolyPhen-2”, a tool that predicts possible impact of a substitution of an amino-acid on the structure and function of a human protein using physical and comparative considerations(5); “SNPs&GO”, a predictor of human disease-related mutations in proteins that considers information from evolutionary informations, gene ontology terms and protein sequence(6); PROVEAN, an alignment-based score which measures the change in sequence similarity of a query sequence to a protein sequence homolog before and after the introduction of an amino acid variation to the query sequence(7). References 1. Ricci R, Arena V, Castri F, et al. Role of p16/INK4a in gastrointestinal stromal tumor progression. Am J Clin Pathol 2004;122:35-43. 2. Yamamoto H, Oda Y, Kawaguchi K, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pathol 2004;28:479-88. 3. Martini M, Santoro L, Familiari P, Costamagna G, Ricci R. Inflammatory fibroid polyp of the gallbladder bearing a platelet-derived growth factor receptor alpha mutation. Arch Pathol Lab Med 2013;137:721-4. 4. Sim NL, Kumar P, Hu J, et al. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 2012;40:W452-7. 5. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248-9. 6. Calabrese R, Capriotti E, Fariselli P, Martelli PL, Casadio R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat 2009;30:1237-44. 7. Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PloS one 2012;7:e46688.